Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
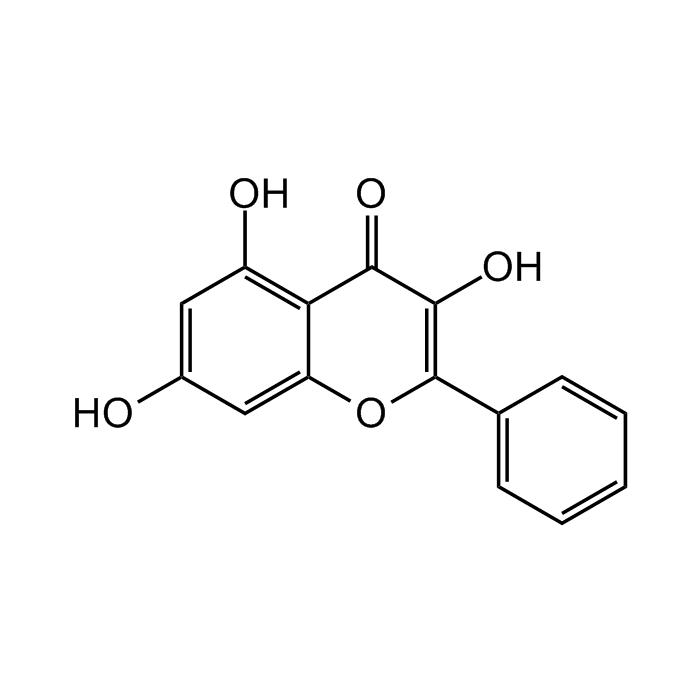
Galangin
| Product Details | |
|---|---|
| Synonyms | 3,5,7-Trihydroxyflavone; Norizalpinin; NSC 407229 |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H10O5 |
| MW | 270.24 |
| CAS | 548-83-4 |
| RTECS | LK9275500 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Light yellow powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or ethanol (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VCCRNZQBSJXYJD-UHFFFAOYSA-N |
| Smiles | OC1=C2C(OC(C3=CC=CC=C3)=C(O)C2=O)=CC(O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Galangin is a flavonoid naturally found in herbs used in traditional medicine. It has diverse biological activities, including potent antioxidant, anti-inflammatory, antiobesity, antidiabetic, anti-cancer, neuroprotective, antiviral and antibacterial properties. Galangin has anti-inflammatory actions related to suppression of signaling through NF-κ/NRLP3 pathway. Galangin induces apoptosis and autophagy in different cancer cells. It is a TRPC5 inhibitor, inhibiting Ca2+ entry into TRPC5 expressing cells. Galangin as a potential DPP-4 inhibitor that improves insulin-stimulated skeletal muscle glucose uptake.
(1) J.J. Meyer, et al.; J. Ethnopharmacol. 56, 165 (1997) | (2) S. Pepeljnjak & I. Kosalec; FEMS Microbiol. Lett. 240, 111 (2004) | (3) H.T. Zhang, et al.; World J. Gastroenterol. 16, 3377 (2010) | (4) M. Wen, et al.; Pharmacology 89, 247 (2012) | (5) W. Zhang, et al.; J. Cell Biochem. 114, 152 (2013) | (6) S. Kumar & K.R. Alagawadi; Pharm. Biol. 51, 607 (2013) | (7) S. Shaikh, et al.; CNS Neurol. Disord. Drug Targets 13, 452 (2014) | (8) Y.C. Jung, et al.; Immunopharmacol. Immunotoxicol. 36, 426 (2014) | (9) J. Naylor, et al.; Br. J. Pharmacol. 173, 562 (2016) | (10) G. Chen, et al.; Int. J. Mol. Sci. 19, E12 (2017) | (11) A.A. Aloud, et al.; Redox Rep. 23, 29 (2018) | (12) Q. Fu, et al.; Mol. Med. Rep. 18, 3619 (2018) | (13) M.E. Kim, et al.; Mol. Cell Biochem. 451, 145 (2019) | (14) P. Kalhotra, et al.; Int. J. Mol. Sci. 20, E1228 (2019)





