Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
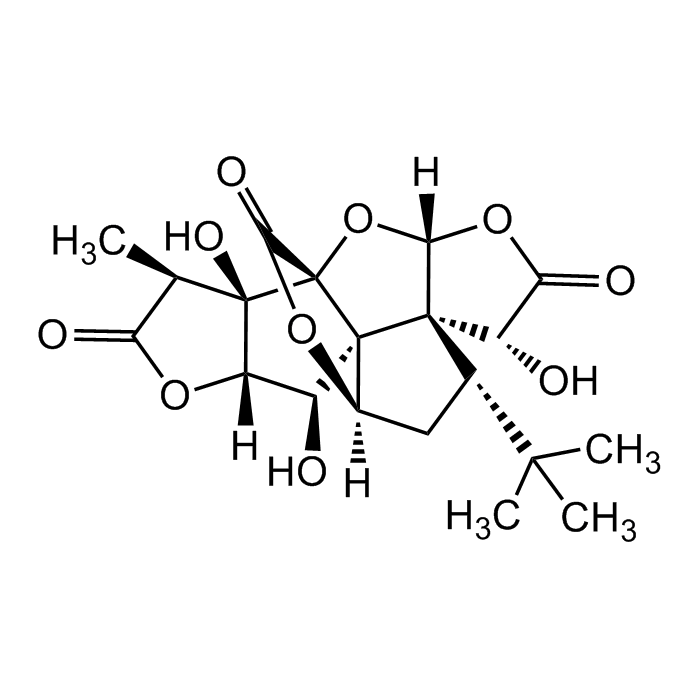
Ginkgolide B
| Product Details | |
|---|---|
| Synonyms | BN-52021 |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H24O10 |
| MW | 424.4 |
| CAS | 15291-77-7 |
| RTECS | KC9949000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO (30mg/ml) or ethanol (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SQOJOAFXDQDRGF-MMQTXUMRSA-N |
| Smiles | O=C1[C@@H](C)[C@]([C@](O1)([H])[C@@H]2O)(O)[C@]([C@]32[C@@]4([H])C[C@@H](C(C)(C)C)[C@]53[C@H]6O)(C(O4)=O)O[C@]5([H])OC6=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Ginkgolide B, a terpenoid extracted from G. biloba leaves, is a potent PAFR antagonist that inhibits platelet aggregation. Platelet-activating factor (PAF) is an important mediator of cell proliferation, angiogenesis, inflammatory response regulation, vasodilation, superoxide formation and platelet aggregation. These cellular effects are mediated through its specific G-protein coupled receptor, PAFR. Ginkgolide B has been shown to have anticancer properties by inducing apoptosis and blocking the cell cycle. Ginkgolide B also demonstrates number of other anti-inflammatory, anti-allergic, antioxidant and neuroprotective effects.
(1) E. Foldes-Filep, et al.; BBRC 148, 1412 (1987) | (2) K. Funkunaga, et al.; J. Biol. Chem. 276, 43025 (2001) | (3) K.M. Maclennan, et al.; Prog. Neurobiol. 67, 235 (2002) | (4) E.L. Kondratskaya, et al.; Brain Res. Bull. 63, 309 (2004) | (5) X. Shi-hai & F. Dian-chun; Chin. Med. J. 120, 922 (2007) | (6) H.J. Cho & K.S. Nam; J. Biochem. Mol. Biol. 40, 678 (2007) | (7) M. Aponte, et al.; Cancer Res. 68, 5839 (2008) | (8) Y.D. Hsuuw, et al.; Ann. N Y Acad. Sci. 1171, 501 (2009) | (9) S. Zhang, et al.; J. Cardiovasc. Pharmacol. 57, 721 (2011) | (10) C. Zhang, et al.; Toxicology 287, 124 (2011) | (11) X. Chu, et al.; Molecules 16, 7634 (2011) | (12) W. Jiang, et al.; Integr. Cancer Ther. 13, NP10-7 (2012) | (13) J.H. Gu, et al.; Eur. J. Pharm. Sci. 47, 652 (2012)





