Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
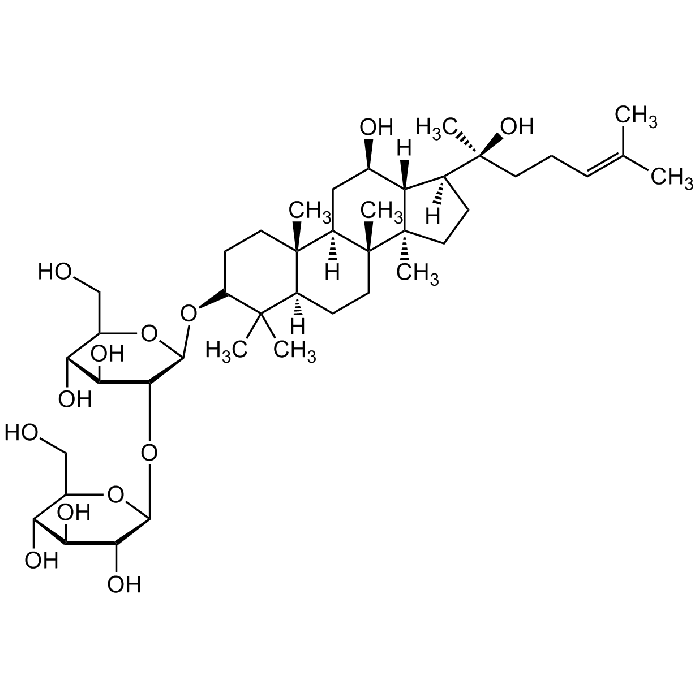
Ginsenoside Rg3
| Product Details | |
|---|---|
| Synonyms | 20(S)-Ginsenoside-Rg3; Rg3; (3β,12β)-12,20-Dihydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside |
| Product Type | Chemical |
| Properties | |
| Formula |
C42H72O13 |
| MW | 785.01 |
| CAS | 14197-60-5 |
| RTECS | LY9537300 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to beige powder. |
| Solubility | Soluble in DMSO (10mg/ml), DMF (10mg/ml) or ethanol (15mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | RWXIFXNRCLMQCD-XRBUSOSMSA-N |
| Smiles | OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2O[C@H]3C(C)(C)[C@@](CC[C@]4(C)[C@]5([H])C[C@@H](O)[C@@]6([H])[C@]4(CC[C@@]6([C@@](C)(O)CC/C=C(C)/C)[H])C)([H])[C@]5(C)CC3)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Ginsenoside Rg3 is a panaxadiol found in white and red P. ginseng. Show a broad range of biological in vitro and in vivo effects, including anticancer, antidiabetic, neuroprotective, antioxidant, anti-hypertensive, and anti-inflammatory actions. The anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of metastasis and angiogenesis, cell cycle arrest, immunomodulatory effects, sensitization to radiation, reducing multidrug resistance and inducing genotoxicity to the cancer cells. Ginsenoside Rg3 has been shown to inhibit the 5-HT3A and α3β4 nACh receptors, the voltage-dependent Ca2+, K+, and Na+ channel currents. It is a scavenger of hydroxyl radicals and downregulated the expression of DNA methyltransferases, reducing global DNA methylation, modifying the methylation of the promoter region of some relevant genes in cancer. It enhances glucose-stimulated insulin secretion and activates AMPK. Ginsenoside Rg3 regulates NF-κB activity and suppresses the NLRP3 inflammasome activation through inhibition of its assembly.
(1) M. Mochizuki, et al.; Biol. Pharm. Bull. 18, 1197 (1995) | (2) N.D. Kim, et al.; Eur. J. Pharmacol. 367, 41 (1999) | (3) H. Rhim, et al.; Eur. J. Pharmacol. 436, 151 (2002) | (4) S. Kim, et al.; BBRC 323, 416 (2004) | (5) Q. Zhang, et al.; BBRC 342, 824 (2006) | (6) M.W. Park, et al.; Biol. Pharm. Bull. 31, 748 (2008) | (7) J.T. Hwang, et al.; Phytother. Res. 23, 262 (2009) | (8) J. Tian, et al.; Phytother. Res. 23, 486 (2009) | (9) X. Wei, et al.; Fitoterapia 83, 636 (2012) | (10) Y.M. Shin, et al.; Mol. Biol. Rep. 40, 269 (2013) | (11) D.G. Kim, et al.; Oncotarget 5, 4438 (2014) | (12) K.J. Son, et al.; Immune Netw. 16, 75 (2016) | (13) H.Y. Sun, et al.; Anticancer Res. 36, 4647 (2016) | (14) M. Sun, et al.; Int. J. Mol. Med. 39, 507 (2017) (Review) | (15) I.S. Lee, et al.; J. Immunol. Res. 2016, 7521601 (2016) | (16) L. Zhang, et al.; Front. Pharmacol. 8, 113 (2017) | (17) M. Nakhjavani, et al.; Medicines 6, E17 (2019) (Review) | (18) Y. Shi, et al.; FASEB J. 34, 208 (2020)





