Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
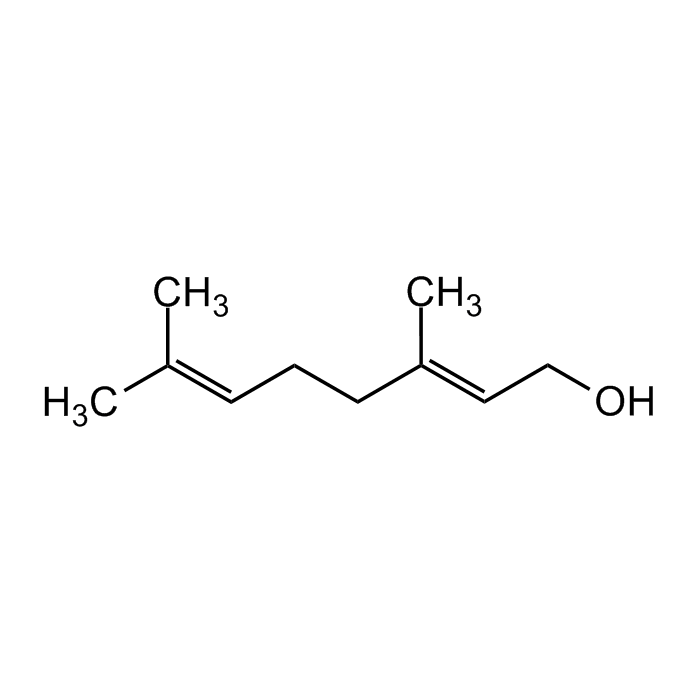
Geraniol
| Product Details | |
|---|---|
| Synonyms | trans-3,7-Dimethyl-2,6-octadien-1-ol; β-Geraniol; (E)-Geraniol; NSC 92793; 7-dimethyl-2E,6-octadien-1-ol |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H18O |
| MW | 154.3 |
| CAS | 106-24-1 |
| RTECS | RG5830000 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥99% (GC) |
| Appearance | Colourless liquid. |
| Solubility | Soluble in methanol or chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | GLZPCOQZEFWAFX-JXMROGBWSA-N |
| Smiles | OC/C=C(CC/C=C(C)\C)\C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Geraniol is a terpene alcohol with a pleasant rose-like aroma, known as an important ingredient in many essential oils, and is used commercially as a fragrance compound in cosmetic and household products. Geraniol has diverse biological activities, including insecticide, antioxidant, anti-inflammatory, anticancer, antimicrobial properties, and hepatoprotective, cardioprotective and neuroprotective effects. Geraniol induces apoptosis in various cancer cells. Formulations containing geraniol have been used as fragrance ingredients and as insecticides in agriculture. Geraniol is produced by the scent glands of honeybees to mark nectar-bearing flowers and locate the entrances to their hives. It is also commonly used as an insect repellent, especially for mosquitoes. This compound can be used as analytical reference material.
(1) G.C. Muller; J. Am. Mosq. Control. Assoc. 24, 154 (2008) | (2) W. Chen & A.M. Viljoen; S. Afr. J. Bot. 76, 643 (2010) (Review) | (3) A.Q. Khan, et al.; Exp. Mol. Pathol. 94, 419 (2013) | (4) M. Jayachandran, et al.; Mol. Cell. Biochem. 398, 39 (2015) | (5) S. Elzinga, et al.; Nat. Prod. Chem. Res. 3, 181 (2015) | (6) S.L. Reis, et al.; Molecules 21, 258 (2016) | (7) F. Qi, et al.; J. Buon. 23, 346 (2018) | (8) Y. Lei, et al.; Planta Med. 85, 48 (2019) (Review) | (9) M.H. Pereira de Lira, et al.; J. Ess. Oil Res. 32, 187 (2020) (Review) | (10) W. Maczka, et al.; Molecules 25, 3303 (2020) (Review)





