Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
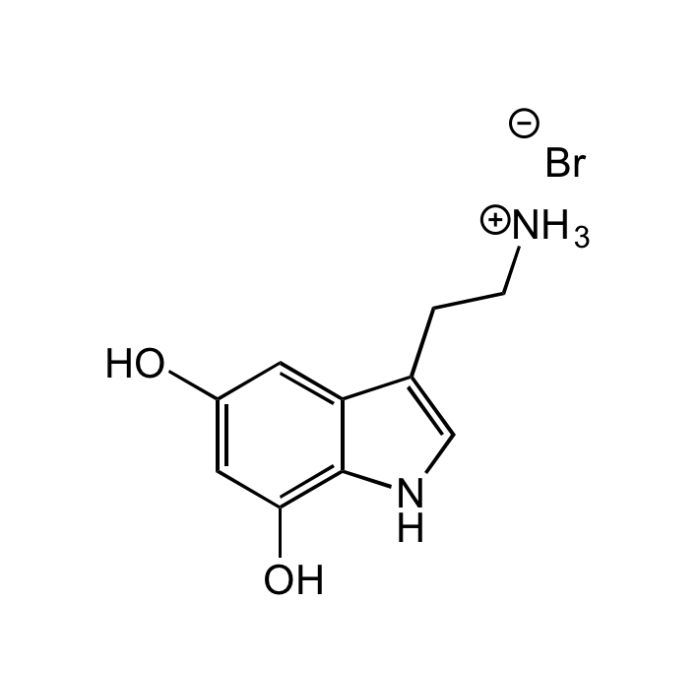
5,7-Dihydroxytryptamine hydrobromide
| Product Details | |
|---|---|
| Synonyms | 5,7-DHT; 3-(2-Aminoethyl)-1H-indole-5,7-diol |
| Product Type | Chemical |
| Properties | |
| Formula | C10H12N2O2 . HBr |
| MW | 192.2 . 80.9 |
| CAS | 31363-74-3 (free base) |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Grey-brown powder. |
| Solubility | Soluble in water. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | KVRZGCBQKAJSBN-UHFFFAOYSA-N |
| Smiles | [Br-].[NH3+]CCC1=CNC2=C(O)C=C(O)C=C12 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Autofluorescent serotonin derivative. Serotonergic neurotoxin, depleting serotonin and norepinephrine. Can be used for the identification of living serotonergic neurons even in the presence of dopaminergic neurons. Its selectively uptaken into 5-hydroxytryptamine neurons. It’s oxidation products are often more potent then the parent product.
(1) H.G. Baumgarten, et al.; Acta Physiol. Scand. Suppl. 391, 1 (1973) | (2) B. Allis & G. Cohen; Eur. J. Pharmacol. 43, 269 (1977) | (3) K. Fuxe, et al.; Ann. N. Y. Acad. Sci. 305, 346 (1978) | (4) M.Z. Wrona, et al.; J. Med. Chem. 29, 499 (1986) | (5) A.K. Sinhababu & R.T. Borchardt; Neurochem. Int. 12, 273 (1988) | (6) K.T. Finnegan, et al.; Brain. Res. 496, 251 (1989) | (7) H. Franke,et al.; Naunyn Schmiedebergs Arch. Pharmacol. 366, 315 (2002)