Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
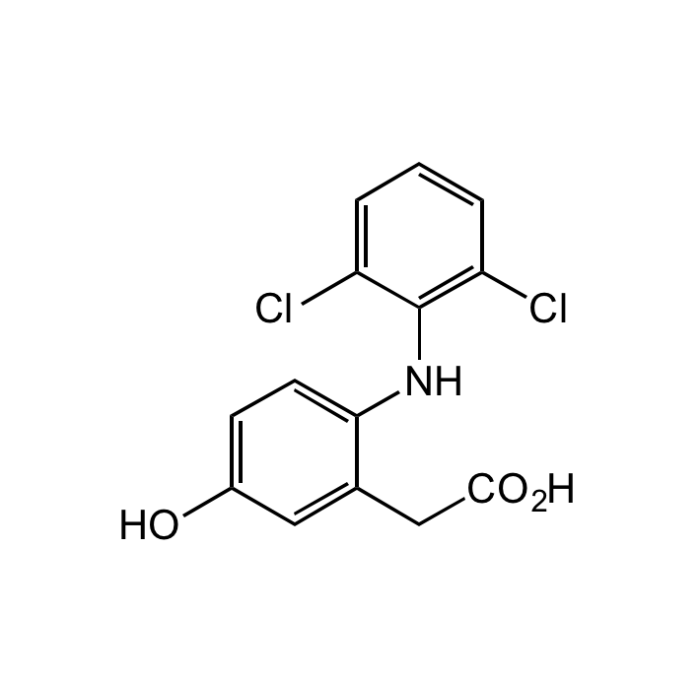
5-Hydroxydiclofenac
| Product Details | |
|---|---|
| Synonyms | 2-[(2,6-Dichlorophenyl)amino]-5-hydroxybenzeneacetic acid; 5-OH-DFN; 5-OH DCF; BRN4199419 |
| Product Type | Chemical |
| Properties | |
| Formula | C14H11Cl2NO3 |
| MW | 312.2 |
| CAS | 69002-84-2 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | Light grey powder. |
| Solubility | Soluble in DMSO or acetone. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VNQURRWYKFZKJZ-UHFFFAOYSA-N |
| Smiles | OC1=CC=C(NC2=C(Cl)C=CC=C2Cl)C(CC(O)=O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Metabolite of Diclofenac, a nonsteroidal anti-inflammatory compound that functions via inhibiton of cyclooxygenase (COX). Diclofenac undergoes biotransformation not only by glucuronidation, but also by cytochrome P450–mediated oxidation to produce 4'-OH-DFN and 5-OH-DFN, which undergo subsequent oxidation to form highly electrophilic benzoquinone imine intermediates. Compound can be used as analytical reference material.
(1) J.R. Kenny, et al.; J. Med. Chem. 47, 2816 (2004) | (2) D.J. Naisbitt, et al.; Toxicol. Lett. 168, 45 (2007) | (3) D. Stülten, et al.; Sci. Total Environ. 405, 310 (2008) | (4) D.J. Waldon, et al.; Chem. Res. Toxicol. 23, 1947 (2010) | (5) J. Surendradoss, et al.; Drug Metab. Disp. 42, 1834 (2014)