Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
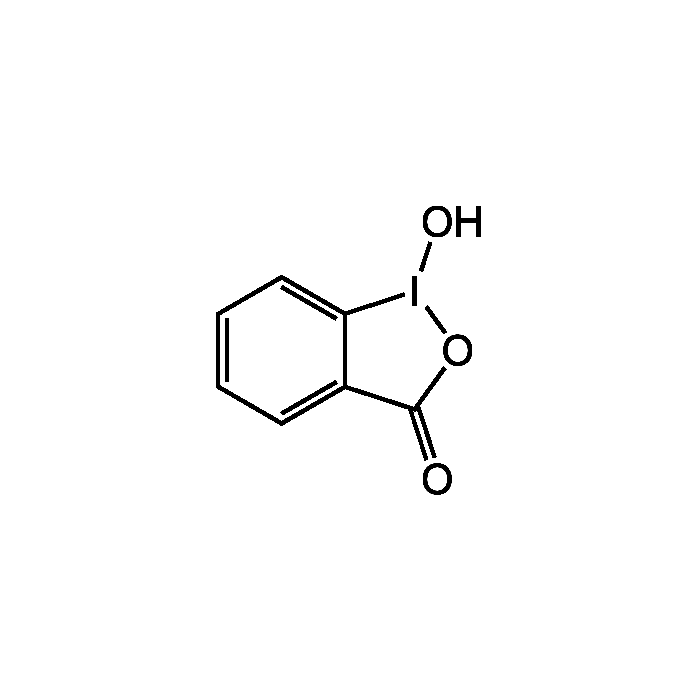
1-Hydroxy-1,2-benziodoxol-3-one
| Product Details | |
|---|---|
| Synonyms | IBA; o-Iodosobenzoic acid; NSC 364374; BR-43089 |
| Product Type | Chemical |
| Properties | |
| Formula | C7H5IO3 |
| MW | 264.1 |
| CAS | 131-62-4 |
| RTECS | DE3850000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in toluene. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AZJIXRYFAZOEMC-UHFFFAOYSA-N |
| Smiles | O[I]1OC(=O)C2=CC=CC=C12 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Heterocyclic iodane with catalytic activity in the hydrolytic cleavage of reactive phosphates, phosphonates and carboxylates. Used as catalyst for the micellar cleavage of activated esters and phosphates and shown to be powerful reagent for the cleavage of phosphates in an aqueous cetyltrimethylammonium chloride (CTAC) micellar medium.
(1) E. Shefter & W. Wolf; J. Pharm. Sci. 54, 104 (1965) | (2) W. Wolf, et al.; J. Pharm. Sci. 54, 329 (1965) | (3) R.A. Moss, et al.; J. Am. Chem. Soc. 105, 681 (1983) | (4) M. Xin & Z. Chen; Synth. Commun. 30, 63 (2000) | (5) V.V. Zhdankin; e-EROS Encycl. Reag. Org. Synth. (2006)