Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
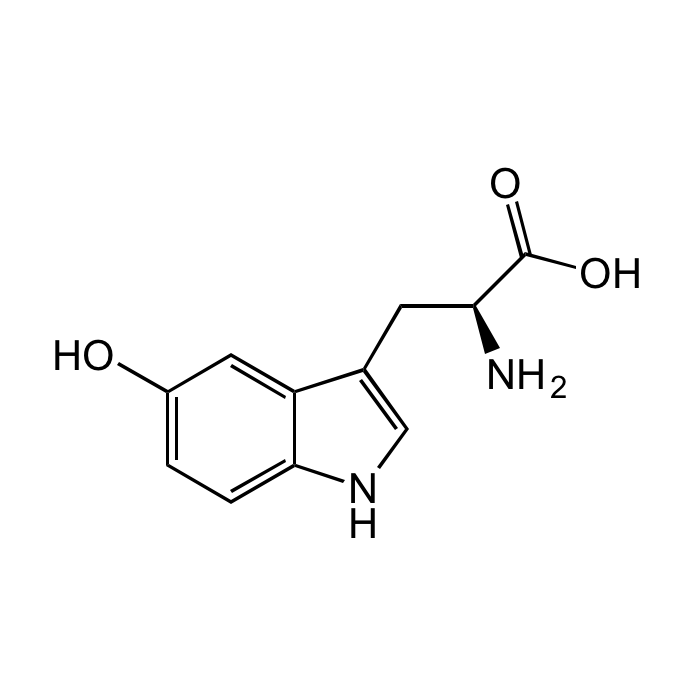
5-Hydroxy-L-tryptophan
| Product Details | |
|---|---|
| Synonyms | Oxitriptan; 5-HTP; L-2-Amino-3-(5-hydroxyindolyl)propionic acid; L-5-HTTP; L-5-Hydroxytryptophan |
| Product Type | Chemical |
| Properties | |
| Formula |
C11H12N2O3 |
| MW | 220.22 |
| CAS | 4350-09-8 |
| RTECS | YN7110000 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | Off-white powder. |
| Solubility | Soluble in water, chloroform or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LDCYZAJDBXYCGN-VIFPVBQESA-N |
| Smiles | OC1=CC=C(NC=C2C[C@H](N)C(O)=O)C2=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
5-hydroxy-L-Tryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin. It is used as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid. It is also used as L-aromatic amino acid decarboxylase substrate. When injected systemically in animals, 5-HTP is converted to serotonin and has both peripheral and central nervous system effects. 5-HTP can also be synthesized by gut microbiota and acts as an activator of the aryl hydrocarbon receptor.
(1) A. Carlsson, et al.; Nature 180, 1200 (1957) | (2) A. Boiardi, et al.; J. Neurol. 225, 41 (1981) | (3) T.C. Birdsall; Altern. Med. Rev. 3, 271 (1998)| (4) A. Martinez, et al.; Curr. Med. Chem. 8, 1077 (2001) | (5) E.H. Turner, et al.; Pharmacol. Ther. 109, 325 (2006) | (6) C.L. Schmid & L.M. Bohn; J. Neurosci. 30, 13513 (2010) | (7) G.V. Sridharan, et al.; Nat. Commun. 5, 5492 (2014) | (8) R. Haberzettl, et al.; Behav. Brain Res. 277, 204 (2015) | (9) J.-A. Mora-Villalobos & A.-P. Zeng; J. Biol. Eng. 12, 3 (2018)





