Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Harmine
As low as
84
CHF
CHF 84.00
In stock
Only %1 left
CDX-H0460-G0011 gCHF 84.00
CDX-H0460-G0055 gCHF 335.00
| Product Details | |
|---|---|
| Synonyms | 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole; Banisterine; Leucoharmine; Telepathine; Yageine; BRN0178813 |
| Product Type | Chemical |
| Properties | |
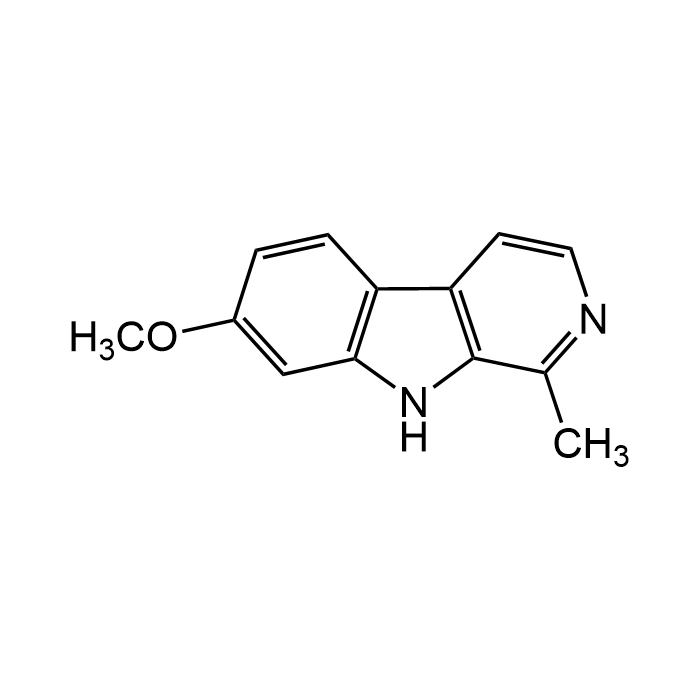
| Formula | C13H12N2O |
| MW | 212.25 |
| CAS | 442-51-3 |
| RTECS | UV0175000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white to light beige powder. |
| Solubility | Soluble in DMSO. Slightly soluble in ethanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | BXNJHAXVSOCGBA-UHFFFAOYSA-N |
| Smiles | CC1=NC=CC2=C1NC3=C2C=CC(OC)=C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Harmine is a fluorescent β-carboline alkaloid. It is a useful fluorescent pH indicator which shows a color change from pH 7.2 (blue fluorescence) to pH 8.9 (yellow fluorescence). It is used with the radioisotope carbon-11 in positron emission tomography neuroimaging to examine its binding to MAO-A. It has several targets and biological activities. It is a potent ATP-competitive and selective inhibitor of DYRK1A, DYRK2 and DYRK3, a competitive and reversible monoamine oxidase inhibitor (MAOI) that reversibly inhibits MAO-A (monoamine oxidase A) but has no effect on MAO-B and a acetylcholinesterase inhibitor (AChEI). It has shown antiviral, antimalarial, anticancer, antidiabetic and osteoclastogenesis activities.
Product References
(1) O.S. Wolfbeis & E. Furlinger; Z. Physik. Chem. 129, 171 (1982) | (2) J.B. Hudson, et al.; Photochem. Photobiol. 43, 21 (1986) | (3) M.G. Gutierrez-Gonzalvez, et al.; Histochemistry 89, 199 (1988) | (4) J. Ishida, et al.; Bioorg. Med. Chem. Lett. 9, 3319 (1999) | (5) H. Waki, et al.; Cell Metab. 5, 357 (2007) | (6) A. Seifert, et al.; FEBS J. 275, 6268 (2008) | (7) S. Nafisi, et al.; J. Photochem. Photobiol. B: Biol. 100, 84 (2010) | (8) T. Adayev, et al.; Arch. Biochem. Biophys. 507, 212 (2011) | (9) D. Shahinas, et al.; Antimicrob. Agents Chemother. 56, 4207 (2012) | (10) P. Wang, et al.; Nat. Med. 21, 383 (2015) | (11) K. Ruben, et al.; PLos One 10, e0132453 (2015) | (12) M.F. Lindberg, et al.; J. Med. Chem. 66, 4106 (2023)





