Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
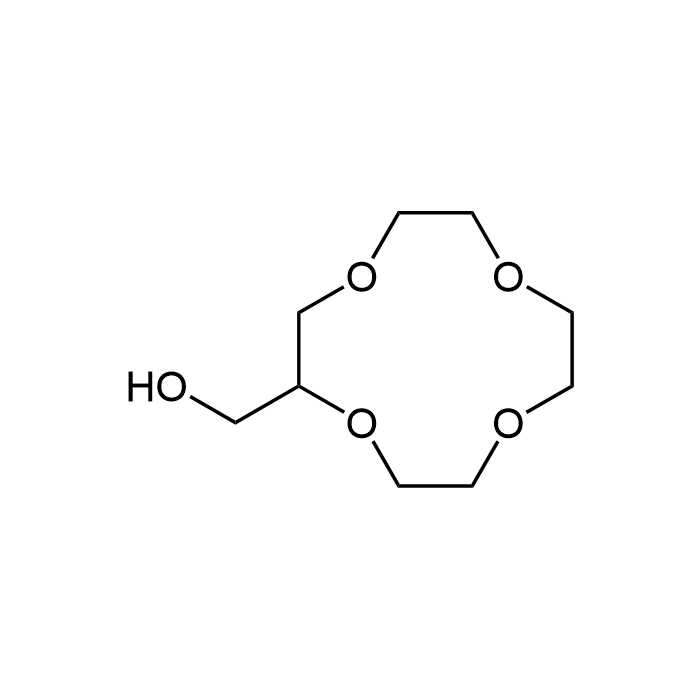
2-Hydroxymethyl-12-crown-4
| Product Details | |
|---|---|
| Synonyms | (12-Crown-4)-2-methanol; 1,4,7,10-Tetraoxacyclododecan-2-methanol |
| Product Type | Chemical |
| Properties | |
| Formula | C9H18O5 |
| MW | 206.24 |
| CAS | 75507-26-5 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (GC) |
| Appearance | Colourless to yellow or viscous liquid. |
| Solubility | Soluble in DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NJIPEIQHUNDGPY-UHFFFAOYSA-N |
| Smiles | OCC1COCCOCCOCCO1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
2-Hydroxymethyl-12-crown-4 is ionophoric and used as an efficient phase transfer catalyst and as a complexing agent with a variety of small cations. This crown ether is a cyclic chemical compound that forms stable complexes with certain cations due to its ring structure containing multiple ether groups. The core structure is a 12-membered ring with 4 oxygen atoms spaced evenly around the ring. This structure is conducive to binding monovalent cations, particularly lithium (Li+), sodium (Na+) and potassium (K+). A hydroxymethyl group (-CH2OH) is attached to the second carbon in the ring. This adds an additional functional group to the crown ether, which can impact its solubility, reactivity, and binding properties. Crown ethers like 2-Hydroxymethyl-12-crown-4 are often used to facilitate the transport of cations across membranes. They can act as phase transfer catalysts, helping to move ionic reactants into organic phases where they can react more readily. These compounds are studied for their ability to form stable complexes with metal ions, which has implications in both inorganic and organic chemistry. 2-Hydroxymethyl-12-crown-4 is used as a bulding block or intermediate for synthesis of probes.
(1) I. Ikeda, et al.; Tetrahedr. Lett. 22, 3615 (1981) | (2) T. Miyazaki, et al.; Bull. Chem. Soc. Japan 55, 2005 (1982) | (3) S. Kitazawa, et al.; JACS 106, 6978 (1984) | (4) M.J. Pugia, et al.; J. Org. Chem. 52, 2617 (1987) | (5) B.P.S. Chauhan & P. Boudjouk; Tetrahedr. Lett. 40, 4123 (1999) | (6) A. Varnek, et al.; J. Chem. Inf. Comput. Sci. 42, 812 (2002) | (7) R. Pankiewicz, et al.; J. Mol. Struct. 749, 128 (2005) | (8) Z. Biyikliouglu & H. Kanteking; Dyes Pigm. 80, 17 (2009) | (9) P. Bruni, et al.; J. Mol. Struct. 919, 328 (2009) | (10) S.J. Warnock, et al.; PNAS 118, e2022197118 (2021) | (11) L. Pang, et al.; Mat. Design 211, 110159 (2021)





