Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
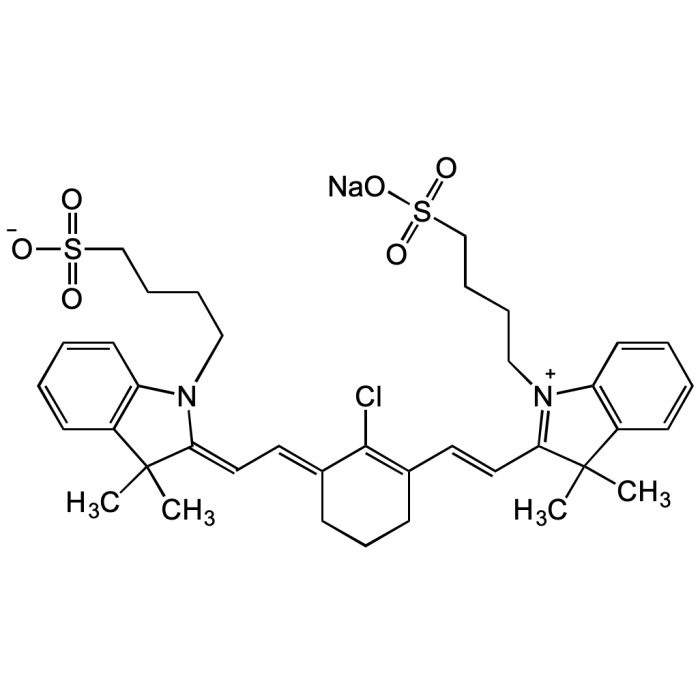
IR-783
| Product Details | |
|---|---|
| Synonyms | 2-[2-[2-Chloro-3-[2-[1,3-dihydro-3,3-dimethyl-1-(4-sulfobutyl)-2H-indol-2-ylidene]-ethylidene]-1-cyclohexen-1-yl]-ethenyl]-3,3-dimethyl-1-(4-sulfobutyl)-3H-indolium hydroxide, inner salt sodium salt |
| Product Type | Chemical |
| Properties | |
| Formula | C38H46ClN2NaO6S2 |
| MW | 749.37 |
| CAS | 115970-66-6 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Dark red to brown powder. |
| Solubility | Soluble in water or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QQIQAVJARACLHE-UHFFFAOYSA-M |
| Smiles | ClC1=C(/C=C/C2=[N+](CCCCS(=O)(O[Na])=O)C3=C(C=CC=C3)C2(C)C)CCC/C1=C\C=C4N(CCCCS(=O)([O-])=O)C(C=CC=C5)=C5C/4(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
IR-783 is a functional laser and near-IR (NIR) dye. Spectral data: Absorption λmax=783nm (MeOH). IR-783 has been shown to be a dye that exhibits imaging, cancer targeting and anticancer properties. Useful optical probe for NIR imaging of tumors, shown to accumulate in the mitochondria and lysosomes of cancer cells. IR-783 inhibits cell viability and induces mitochondrial apoptosis in human breast cancer cells and induces cell proliferation in a dose- and time-dependent manner by inducing cell cycle arrest at the G0/G1 phase.
(1) K. Leung; Mol. Imag. Contrast Agent Datab. (2010) | (2) X. Yang, et al.; Clin. Cancer Res. 16, 2833 (2010) | (3) K. Leung; Mol. Imag. Contrast Agent Datab. (2011) | (4) X. Yang, et al.; J. Urol. 189, 702 (2013) | (1) C. Shao, et al.; PLoS One 9, e88967 (2014) | (6) I. Semenenko, et al.; Front. Pharmacol. 7, 426 (2016) | (7) Q. Tang, et al.; J. Cell Mol. Med. 22, 4474 (2018) | (8) P. Li, et al.; Int. J. Oncol. 55, 415 (2019)





