Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
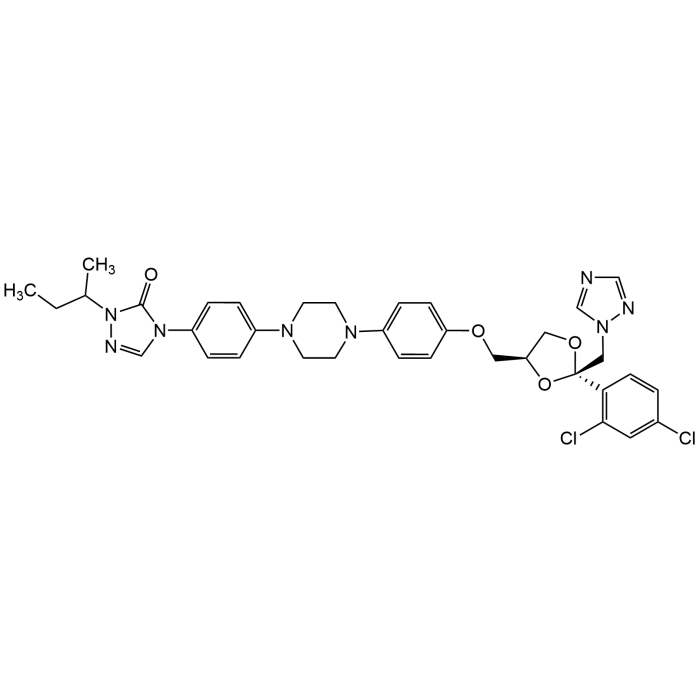
Itraconazole
As low as
116
CHF
CHF 116.00
In stock
Only %1 left
CDX-I0072-M100100 mgCHF 116.00
| Product Details | |
|---|---|
| Synonyms | Oriconazole; R 51211; Triasporin; cis-Itraconazole; Fungitraxx |
| Product Type | Chemical |
| Properties | |
| Formula | C35H38Cl2N8O4 |
| MW | 705.63 |
| CAS | 84625-61-6 |
| RTECS | XZ5481000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in chloroform and DMSO. Slightly soluble in ethanol or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VHVPQPYKVGDNFY-ZPGVKDDISA-N |
| Smiles | O=C(N(C(CC)C)N=C1)N1C2=CC=C(N3CCN(C4=CC=C(OC[C@@H]5O[C@@](CN6N=CN=C6)(C7=C(Cl)C=C(Cl)C=C7)OC5)C=C4)CC3)C=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Itraconazole is a broad-spectrum triazole antifungal agent widely used in clinical and research applications for its ability to inhibit fungal ergosterol synthesis by targeting the cytochrome P450 enzyme lanosterol 14α-demethylase and compromising the cell membrane, which leads to its fungicidal activity. It is effective against a range of pathogenic fungi, including Aspergillus, Candida, and Histoplasma species, making it valuable for studying antifungal mechanisms and resistance. Beyond its antifungal properties, Itraconazole has anti-angiogenic and Hedgehog pathway-inhibiting effects and has gained interest in cancer research. Itraconazole has inhibitory activity on Smoothened (SMO) at different binding site to cyclopamine, thereby disrupting the signaling in Hedgehog pathway. It has been extensively investigated for its potential repurposing across various cancers, such as basal cell carcinoma, prostate cancer, non-small cell lung cancer, breast cancer, medulloblastoma, glioblastoma and pancreatic cancer.
Product References
(1) J. Van Cutsem, et al.; Antimicrob. Agents Chemother. 26, 527 (1984) | (2) H. Vanden Bossche, et al.; Antimicrob. Agents Chemother. 37, 2101 (1993) | (3) J.M. Zuckerman & A.R. Tunkel; Infect. Control Hosp. Epidemiol. 15, 397 (1994) | (4) C.R. Chong, et al.; ACS Chem. Biol. 2, 263 (2007) | (5) J. Kim, et al.; Cancer Cell 17, 388 (2010) | (6) J. Kim, et al.; Cancer Cell 23, 23 (2013) | (7) J.R. Pace, et al.; J. Med. Chem. 59, 3635 (2016) | (8) M. Wahid, et al.; Crit. Rev. Oncol. Hematol. 98, 235 (2016) | (9) X. Wei, et al.; J. Cancer Res. Clin. Oncol. 146, 297 (2020) | (10) C.L. Li, et al.; Biomed. Pharmacother. 154, 113616 (2020) | (11) A.M. Kulkarni, et al.; Biochim. Biophys. Acta Rev. Cancer 1880, 189279 (2025)





