Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Irbesartan
| Product Details | |
|---|---|
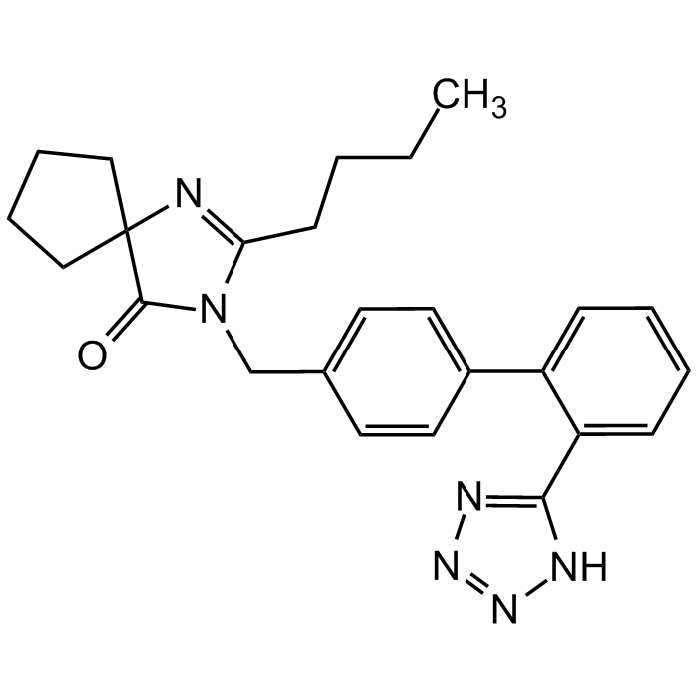
| Synonyms | 2-Butyl-3-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one; Avapro; BMS-186295; SR-47436 |
| Product Type | Chemical |
| Properties | |
| Formula |
C25H28N6O |
| MW | 428.5 |
| CAS | 138402-11-6 |
| RTECS | HM2950270 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YOSHYTLCDANDAN-UHFFFAOYSA-N |
| Smiles | O=C1C2(CCCC2)N=C(CCCC)N1CC(C=C3)=CC=C3C4=CC=CC=C4C5=NN=NN5 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Irbesartan is an orally available angiotensin II type 1 (AT1) receptor antagonist (IC50 = 1.3 nM) with antihypertensive activity. It binds to AT1 receptor with >5000 times higher affinity compared to AT2 receptor. Irbesartan also elicits selective peroxisome proliferator-activated receptor γ (PPARγ)-modulating activity and exhibits metabolic, anti-inflammatory and antioxidative properties. Irbesartan inhibits the receptor for advanced glycation end-products (RAGE)/high mobility group box 1 (HMGB1) axis and maybe useful in prevention and treatment of various diseases. Formulations containing irbesartan have been used, alone and in combination with diuretics, in the treatment of hypertension.
(1) L. Ruilope; J. Hypertens. Suppl. 15, S15 (1997) (Review) | (2) M. Burnier; Circulation 103, 904 (2001) | (3) A.H. Gradman; J. Human Hypert. 16, S9 (2002) | (4) K. Kikuchi, et al.; Int. J. Mol. Sci. 14, 18899 (2013) (Review) | (5) Z.Z. Zhang, et al.; J. Transl. Med. 11, 229 (2013) | (6) I. Taguchi, et al.; Hypertens. Res. 36, 608 (2013) | (7) I.T. Abdel-Raheem, et al.; Fundam. Clin. Pharmacol. 29, 286 (2015) | (8) J. Zhong, et al.; Int. Immunopharmacol. 42, 176 (2017) | (9) I.A. Darwish, et al.; Prof. Drug Subst. Excip. Rel. Meth. 46, 185 (2021) (Review)





