Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
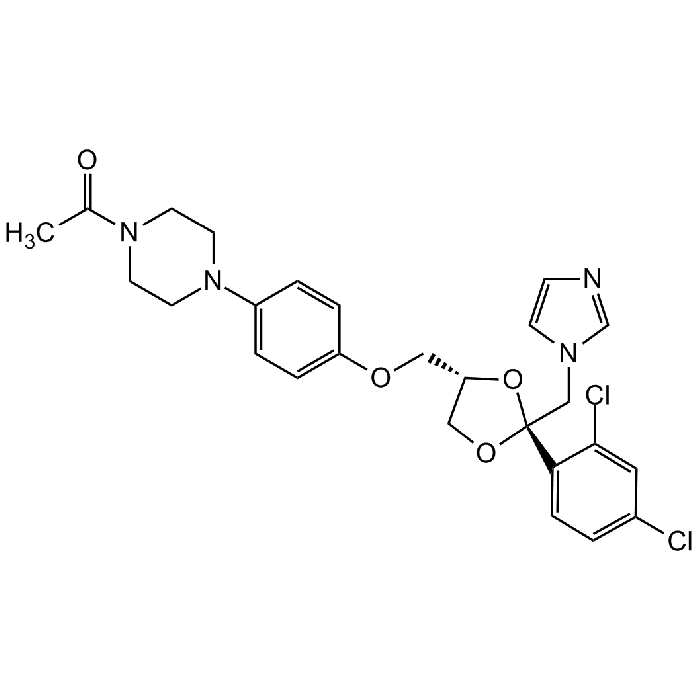
Ketoconazole
| Product Details | |
|---|---|
| Synonyms | (±)-cis-1-Acetyl-4-(4-[(2-[2,4-dichlorophenyl]-2-[1H-imidazol-1-ylmethyl]-1,3-dioxolan-4-yl)-methoxy]phenyl)piperazine; R 41400; NSC 317629; BRN 4303081 |
| Product Type | Chemical |
| Properties | |
| Formula |
C26H28Cl2N4O4 |
| MW | 531.43 |
| CAS | 65277-42-1 |
| RTECS | TK7912300 |
| Purity Chemicals | 99.0-101.0% (EP, Titration) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (30mg/ml) or ethanol (20mg/ml) or methanol (20mg/ml). |
| Identity | Determined by IR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XMAYWYJOQHXEEK-OZXSUGGESA-N |
| Smiles | CC(N(CC1)CCN1C2=CC=C(OC[C@H]3CO[C@](CN4C=NC=C4)(C5=CC=C(Cl)C=C5Cl)O3)C=C2)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Ketoconazole is a broad-spectrum triazole antifungal agent that has activity against C. albicans, C. krusei, C. tropicalis, C. glabrata, C. parapsilosis, C. neoformans and A. fumigatus strains (IC50s = 0.031-8 µg/ml). It inhibits the fungal cytochrome P450 (CYP) isoform CYP51, also known as lanosterol 14α-demethylase, which arrests ergosterol biosynthesis at the fungal membrane. This interaction inhibits ergosterol synthesis and results in increased fungal cellular permeability. Ketoconazole also inhibits human CYP3A4 (IC50 = 0.54 µM). Formulations containing ketoconazole have been used in the treatment of fungal infections. Ketoconazole blocks in humans activity of several enzymes necessary for the conversion of cholesterol to steroid hormones such as testosterone and cortisol. It has been shown to inhibit cholesterol side-chain cleavage enzyme, which converts cholesterol to pregnenolone, 17α-hydroxylase and 17,20-lyase, which convert pregnenolone into androgens, and 11β-hydoxylase, which converts 11-deoxycortisol to cortisol. All of these enzymes are mitochondrial cytochrome p450 enzymes. Based on these antiandrogen and antiglucocorticoid effects, ketoconazole has been used with some success as a second-line treatment for certain forms of advanced prostate cancer and for the suppression of glucocorticoid synthesis in the treatment of Cushing's syndrome. Ketoconazole displays potent anti-metastatic, anti-neoplastic, and anti-psoriatic activities and acts as an inhibitor of 5-lipoxygenase (5-LO) and thromboxane synthase activities.
(1) D.S. Loose, et al.; J. Clin. Invest. 71, 1495 (1983) | (2) D.S. Loose, et al.; J. Clin. Invest. 72, 404 (1983) | (3) M. Borgers, et al.; Am. J. Med. 74, 2 (1983) (Review) | (4) J.H. Van Tyle; Pharmacotherapy 4, 343 (1984) (Review) | (5) M. Ayub & M.J. Levell; J. Steroid Biochem. 32, 515 (1989) | (6) C. Eil; Horm. Metab. Res. 24, 367 (1992) | (7) R. Dumaine, et al.; J. Pharmacol. Exp. Ther. 286, 727 (1998) | (8) A. DeVries, et al.; Pharmacotherapy 18, 581 (1998) (Review) | (9) S. Dilmaghanian, et al.; Chirality 16, 79 (2004) | (10) D.J. Greenblatt, et al.; Xenobiotica 40, 713 (2010) (Review) | (11) V. Patel, et al.; Nat. Rev. Urol. 15, 643 (2018) (Review)





