Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
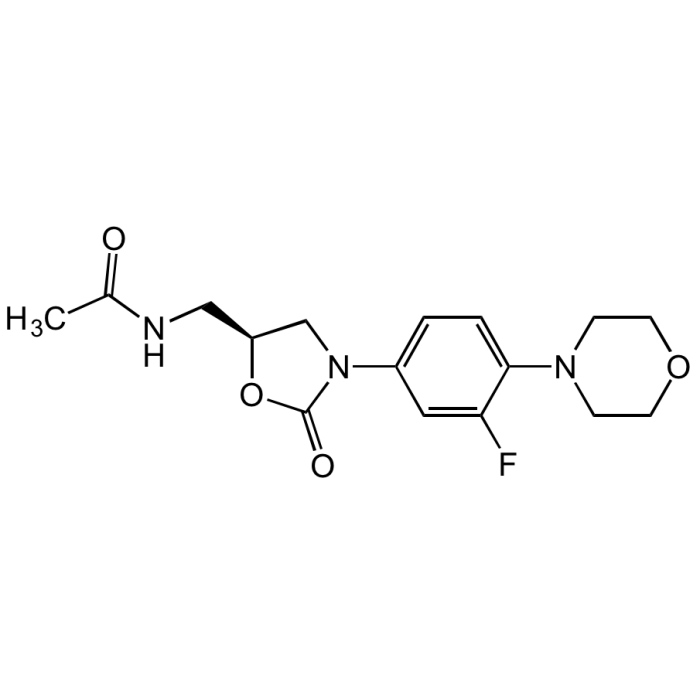
Linezolid
| Product Details | |
|---|---|
| Synonyms | N-[[(5S)-3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide; PNU-100766; U-100766 |
| Product Type | Chemical |
| Properties | |
| Formula | C16H20FN3O4 |
| MW | 337.35 |
| CAS | 165800-03-3 |
| RTECS | AC2720000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF or chloroform. Slightly soluble in ethanol (1mg/ml) methanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | TYZROVQLWOKYKF-ZDUSSCGKSA-N |
| Smiles | CC(NC[C@H]1CN(C2=CC=C(N3CCOCC3)C(F)=C2)C(O1)=O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Linezolid is a synthetic oxazolidinone antibiotic with activity against a wide range of Gram-positive bacteria. Displays potent antibacterial activity against a variety of multidrug-resistant gram-positive microbes in vitro and in vivo, including resistant strains of several species, such as methicillin-resistant S. aureus (MRSA), penicillin-resistant pneumococci, vancomycin-resistant enterococci, various anaerobic bacteria, and several mycobacteria and streptococci (MICs = 2-4mg/ml). Linezolid inhibits protein synthesis by binding to domain V of the 23S ribosomal RNA of the 50S subunit of bacterial ribosomes and preventing the formation of a functional 70S initiation complex, thus inhibiting bacterial mRNA translation. Linezolid is also a weak, reversible, nonselective inhibitor of monoamine oxidase.
(1) C.W. Ford, et al.; Antimicrob. Agents Chemother. 40, 1508 (1996) | (2) S.J. Brickner, et al.; J. Med. Chem. 39, 673 (1996) | (3) R.N. Jones, et al.; Antimicrob. Agents Chemother. 40, 720 (1996) | (4) D. Clemett & A. Markham; Drugs 59, 815 (2000) (Review) | (5) H.B. Fung, et al.; Clin. Ther. 23, 356 (2001) (Review) | (6) C.W. Ford, et al.; Curr. Drug Targets Infect. Disord. 1, 181 (2001) (Review) | (7) D.M. Livermore; J. Antimicrob. Chemother. 51, ii9 (2003) (Review) | (8) S.J. Brickner, et al.; J. Med. Chem. 51, 1981 (2008) (Review) | (9) K.L. Leach, et al.; Ann. N. Y. Acad. Sci. 1222, 49 (2011) (Review) | (10) S. Ager & K. Gould; Infect. Drug Resist. 5, 87 (2012) | (11) B.A. Diep, et al.; Curr. Ther. Res. Clin. Exp. 73, 86 (2012) (Review) | (12) L.A. Pauchard, et al.; PLoS One 12, e0187187 (2017)





