Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
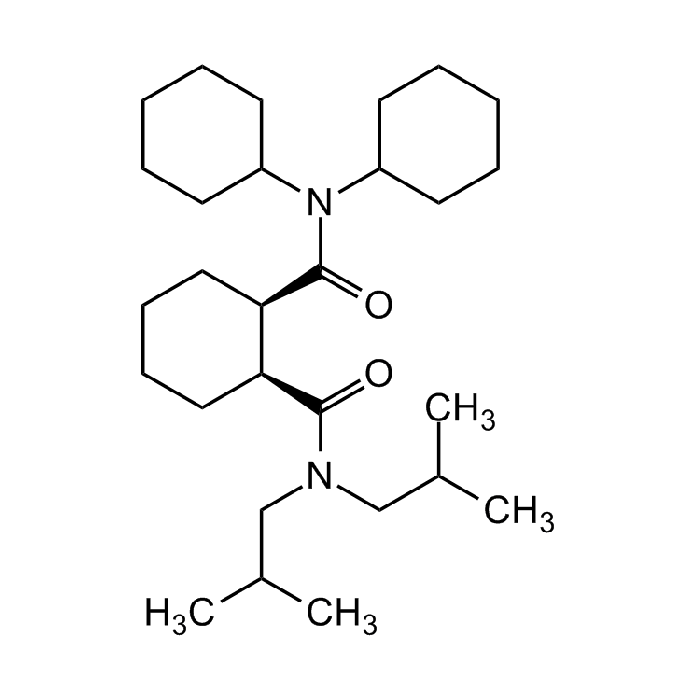
Litihium Ionophore III
| Product Details | |
|---|---|
| Synonyms | ETH 1810; N,N-Dicyclohexyl-N',N'-diisobutyl-cis-cyclohexane-1,2-dicarboxamide |
| Product Type | Chemical |
| Properties | |
| Formula |
C28H50N2O2 |
| MW | 446.71 |
| CAS | 99281-50-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in chlorofrom or DCM. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QGKITQHJONXIQV-IZZNHLLZSA-N |
| Smiles | O=C(N(C1CCCCC1)C2CCCCC2)[C@H]3[C@@H](C(N(CC(C)C)CC(C)C)=O)CCCC3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Lithium ionophore III (ETH 1810) has been used in liquid membrane electrodes for the determination of Li concentration in blood. It is a neutral non-cyclic Li(+)-selective ionophore, which can transport lithium and other monovalent cations, across lipid bilayer membranes, forming 2:1 ionophore:ion membrane-permeating species. The selectivity is Li+ > Na+ > K+ > Cs+. ETH 1810 was also found to be capable of acting as a carrier of biogenic amines and related molecules, with the following selectivity sequence: Tryptamine > Phenylethylamine > Tyramine > Serotonin > Li+ > NH+4 > Dopamine. Protons at physiological concentrations do not interfere with the lithium transport.
(1) E. Metzger et al. Anal. Chem. 59, 1600, (1987) | (2) A. Zeevi & R. Margalit; J. Membr. Biol. 121, 133 (1991) | (3) A. Zeevi & R. Margalit; Arch. Biochem. Biophys. 298, 84 (1992) | (4) E. Bakker & E. Pretsch; Anal. Chem. 70, 295 (1998)





