Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
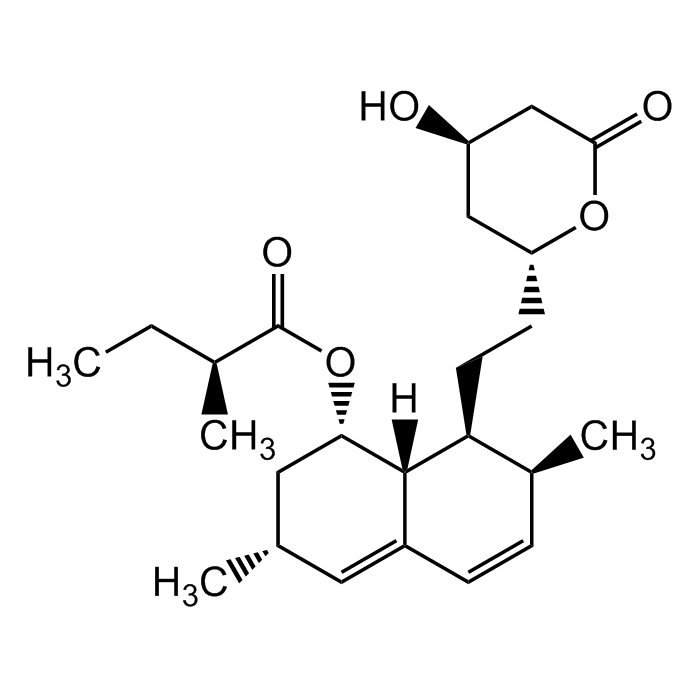
Lovastatin
| Product Details | |
|---|---|
| Synonyms | Mevilonin; MK-803; 6-α-Methylcompactin; BRN 3631989 |
| Product Type | Chemical |
| Properties | |
| Formula |
C24H36O5 |
| MW | 404.54 |
| CAS | 75330-75-5 |
| RTECS | EK7907000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white crystalline powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (15mg/ml) or ethanol (20mg/ml). Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PCZOHLXUXFIOCF-BXMDZJJMSA-N |
| Smiles | C[C@H]1C=CC2=C[C@H](C)C[C@H](OC([C@@H](C)CC)=O)[C@]2([H])[C@H]1CC[C@H]3OC(C[C@H](O)C3)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate. Lovastatin is a prodrug, which is hydrolyzed in vivo to the active β-hydroxy acid open ring form. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA. Lovastatin is an effective anti-hypercholesterolemic agent widely used as a lipid-lowering drug. In addition to lowering blood lipid levels, Lovastatin also has been shown to have anticancer, neuroprotective, anti-inflammatory, antiviral and antibacterial properties.
(1) A.W. Alberts, et al.; PNAS 77, 3957 (1980) | (2) M. Jakobisiak, et al.; PNAS 88, 3628 (1991) | (3) P. Negre-Aminou, et al.; Biochim. Biophys. Acta 1345, 259 (1997) | (4) B. Kwak, et al.; Nat. Med. 6, 1399 (2000) | (5) C. Wojcik, et al.; Int. J. Biochem. Cell Biol. 32, 957 (2000) | (6) L.J. Raggatt & N.C. Partridge; Drugs 62, 2185 (2002) (Review) | (7) W.W. Wong, et al.; Leukemia 16, 508 (2002) | (8) K.K. Chan, et al.; Clin. Cancer Res. 9,10 (2003) | (9) L.M. Blanco-Colio, et al.; Kidney Int. 63, 12 (2003) | (10) J.A. Tobert; Nat. Rev. Drug Discov. 2, 517 (2003) (Review) | (11) V. Chopra, et al.; Cardiovasc. Drugs Ther. 21, 161 (2007) | (12) Z. Xiong, et al.; Food Chem. Toxicol. 131, 110585 (2019) (Review)





