Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Murexide
| Product Details | |
|---|---|
| Synonyms | 5,5'-Nitrilodibarbituric acid monoammonium salt; Ammonium purpurate |
| Product Type | Chemical |
| Properties | |
| Formula | C8H8N6O6 |
| MW | 284.19 |
| CAS | 3051-09-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (UV) |
| Appearance | Dark red to purple powder. |
| Solubility | Soluble in DMSO. Slightly soluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | CKUIXUMHBVWXEB-UHFFFAOYSA-N |
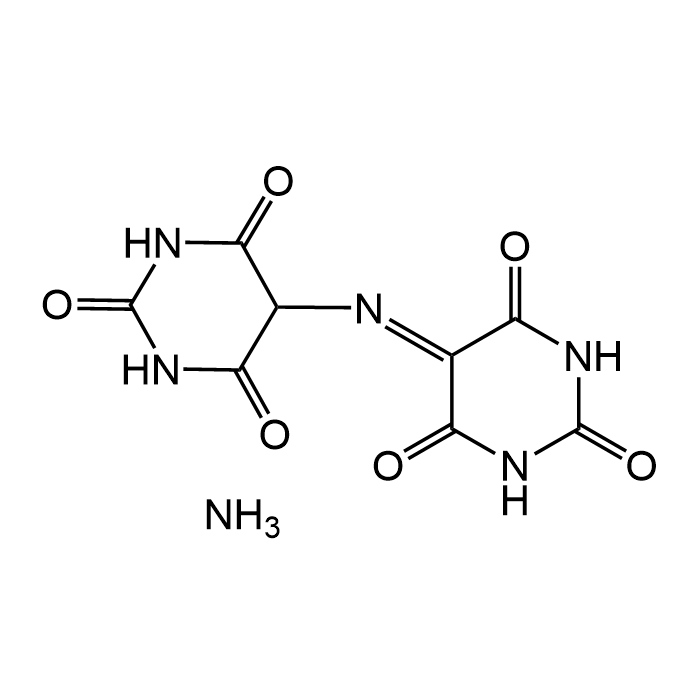
| Smiles | O=C(NC(NC1=O)=O)C1/N=C2C(NC(NC/2=O)=O)=O.N |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Murexide is commonly used as an indicator in complexometric titrations, especially for Ca(II), Co(II), Cu(II), Ni(II), Cd(II), Pb(II), and rare earth metal ions. In these titrations, Murexide changes color when it forms a complex with metal ions, which helps in determining the endpoint of the titration. Murexide is slightly soluble in water. The solution is yellow in strong acidic conditions, reddish-purple in weak acidic conditions, and bluepurple in alkaline conditions. It is also a colorimetric reagent for calcium and rare earth metals and used in spectrophotometric analysis due to its distinct absorption properties. Murexide is used for the histochemical staining of calcium in the skeleton and to measure the dissociation rates of Eu(II)-containing cryptates. Historically, Murexide was used as a dye for textiles due to its vibrant color.
(1) A. Scarpa; Meth. Enzymol. 24, 343 (1972) | (2) R. Docampo, et al.; J. Biol. Chem. 258, 14920 (1983) | (3) M. Shamsipur, et al.; Talanta 36, 773 (1989) | (4) J. Torreilles, et al.; Biochimie 71, 1231 (1989) | (5) M. Shamsipur, et al.; Talanta 36, 1300 (1989) | (6) M. Shamsipur & N. Alizadeh; Talanta 39, 1209 (1992) | (7) S. Jain & P. Gupta-Bhaya; Talanta 39, 1647 (1992) | (8) N. Alizadeh & M. Shamsipur; Talanta 40, 503 (1993) | (9) K. Grudpan, et al.; Talanta 46, 1245 (1998) | (10) X. Yi, et al.; Chemistry 20, 1569 (2014) | (11) K. Kargosha, et al.; J. Fluoresc. 24, 855 (2014) | (12) C.U. Lenora, et al.; Inorg. Chem. 59, 86 (2020)





