Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
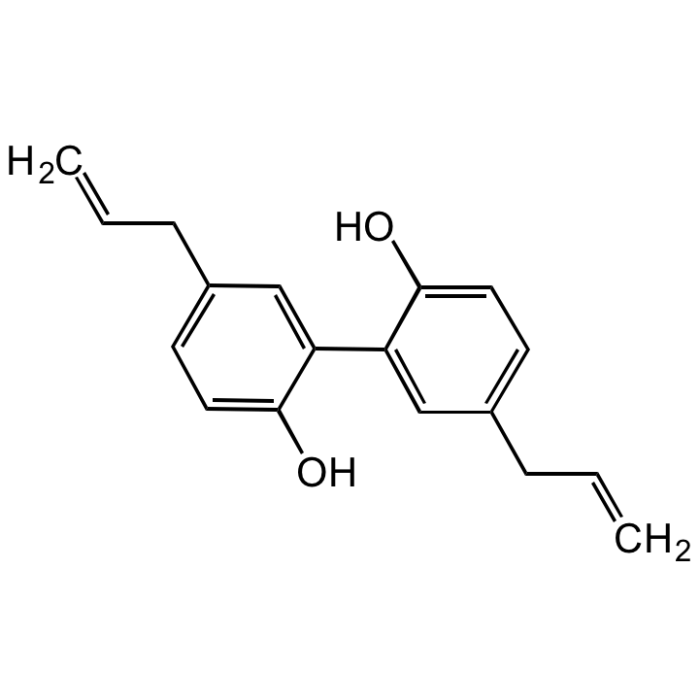
Magnolol
| Product Details | |
|---|---|
| Synonyms | 5,5'-Diallyl-2,2'-biphenyldiol; NSC 293099 |
| Product Type | Chemical |
| Properties | |
| Formula | C18H18O2 |
| MW | 266.33 |
| CAS | 528-43-8 |
| RTECS | DV5105500 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in ethanol (1mg/ml), methanol (1mg/ml), DMSO (10mg/ml) or DMF (10mg/ml). Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VVOAZFWZEDHOOU-UHFFFAOYSA-N |
| Smiles | OC1=CC=C(CC=C)C=C1C2=CC(CC=C)=CC=C2O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Magnolol is a bioactive compound isolated from the bark of M. officinalis that has been used in Asian traditional medicine for the treatment of anxiety, sleep disorders, and allergic diseases. Magnolol is an antifungal, antibacterial and antioxidant compound. It demonstrates anti-inflammatory activity by interferring with NF-κB signalling and NLRP3 inhibition. Magnolol can activate cannabinoid (CB) receptors, behaving as a partial agonist with selectivity for the peripheral CB2 subtype versus central CB1. It been identified as modulators of the GABAA receptors in vitro. It is a potent antitumor and antiangiogenic compound. Shown to inhibit HIF-1α/VEGF signalling, inhibiting invasion and metastasis through MMP2/MMP9 and inhibiting microtubule polymerization. Induces apoptosis through EGFR/PI3K/Akt pathway. Shows anti-diabetic/anti-obesity activity targeting nuclear receptors retinoic X receptor α (RXRα) and peroxisome proliferator activated receptor γ (PPARγ). In addition promotes thermogenesis.
(1) K. Watanabe, et al.; Planta Med. 49, 103 (1983) | (2) J.P. Wang, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 346, 707 (1992) | (3) Y. Maruyama, et al.; J. Nat. Prod. 61, 135 (1998) | (4) K.H. Bang, et al.; Arch. Pharm. Res. 23, 46 (2000) | (5) K.Y. Ho, et al.; Phytother. Res. 15, 139 (2001) | (6) J. Ai, et al.; Pharmacology 63, 34 (2001) | (7) K. Ikeda & H. Nagase; Biol. Pharm. Bull. 25, 1546 (2002) | (8) K. Ikeda, et al.; Phytother. Res. 17, 933 (2003) | (9) J. Park, et al.; Eur. J. Pharmacol. 496, 189 (2004) | (10) A.K. Tse, et al.; Mol. Immunol. 44, 2647 (2007) | (11) D.H. Lee, et al.; J. Cell Biochem. 106, 1113 (2009) | (12) E.S. Hwang & K.K. Park; Biosci. Biotechnol. Biochem. 74, 961 (2010) | (13) H. Zhang, et al.; PLoS One 6, e28253 (2011) | (14) V. Rempel, et al.; ACS Med. Chem. Lett. 4, 41 (2013) | (15) M.C. Chen, et al.; Biochem. Pharmacol. 85, 1278 (2013) | (16) Y. Sakaue, et al.; Microbiol. Immunol. 60, 10 (2016) | (17) J. Shen, et al.; Cell Physiol. Biochem. 42, 1789 (2017) | (18) F. Huang, et al.; Mol. Med. Rep. 16, 4817 (2017) | (19) H.A. Parray, et al.; Nutrition 50, 82 (2018)





