Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Marbofloxacin
As low as
97
CHF
CHF 97.00
In stock
Only %1 left
CDX-M0172-M100100 mgCHF 97.00
| Product Details | |
|---|---|
| Synonyms | 9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-piperazino)-7-oxo-7H-pyrido[1,2,3-ij][1,2,4]benzoxadiazine-6-carboxylic acid; Marbocyl; Zeniquin; Aurizon; CID 60651 |
| Product Type | Chemical |
| Properties | |
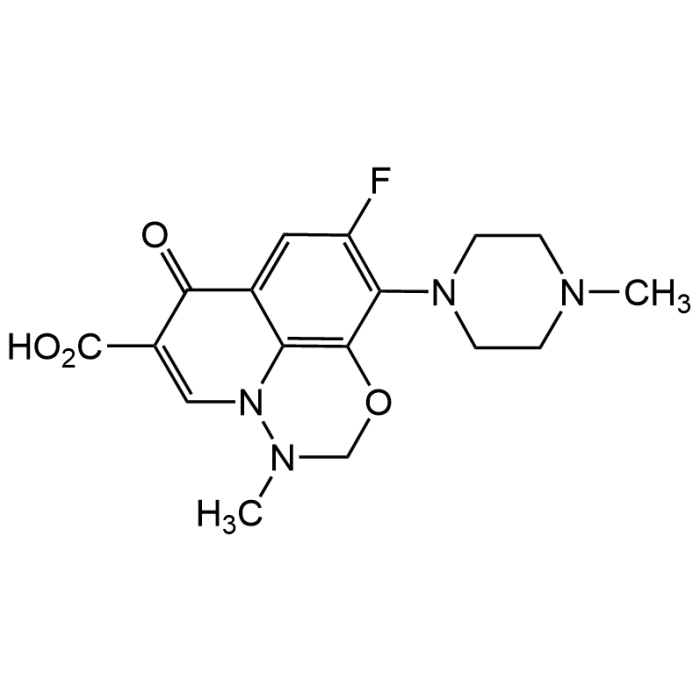
| Formula | C17H19FN4O4 |
| MW | 362.36 |
| CAS | 115550-35-1 |
| RTECS | UU8815140 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥ 98% (HPLC) |
| Appearance | White to beige to brown powder. |
| Solubility | Soluble in DMF (20mg/ml) or DMSO (20 mg/ml). Slightly soluble in ethanol (1 mg/ml) or PBS (1mg/ml). |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | BPFYOAJNDMUVBL-UHFFFAOYSA-N |
| Smiles | O=C1C=2C3=C(C(=C(F)C2)N4CCN(C)CC4)OCN(C)N3C=C1C(O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Marbofloxacin is a broad-spectrum fluoroquinolone antibiotic active against Gram-negative, Gram-positive and Mycoplasma spp. bacteria. It acts by inhibiting the activity of DNA gyrase and topoisomerase IV, enzymes fundamental to the replication and transcription of bacterial DNA. Thus, the inhibition of these enzymes leads to the death of the bacteria. Marbofloxacin has also been shown to exhibit antileishmanial activity in a canine model. Marbofloxacin has low toxicity and limited development of bacterial resistance. Compound can be used as analytical reference material.
Product References
(1) M. Spreng, et al.; J. Vet. Pharmacol. Ther. 18, 284 (1995) | (2) A.A. Ferran, et al.; Vet. Microbiol. 148, 292 (2011) | (3) J. Shen, et al.; Acta Crystallogr. 68, 998 (2012) | (4) C. Pineda, et al.; PLoS One 12, e0185981 (2017) | (5) Z. Lei, et al.; Front. Pharmacol. 8, 1 (2017) | (6) T.L.A. da Silva, et al.; J. AOAC Int. 105, 456 (2022)





