Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
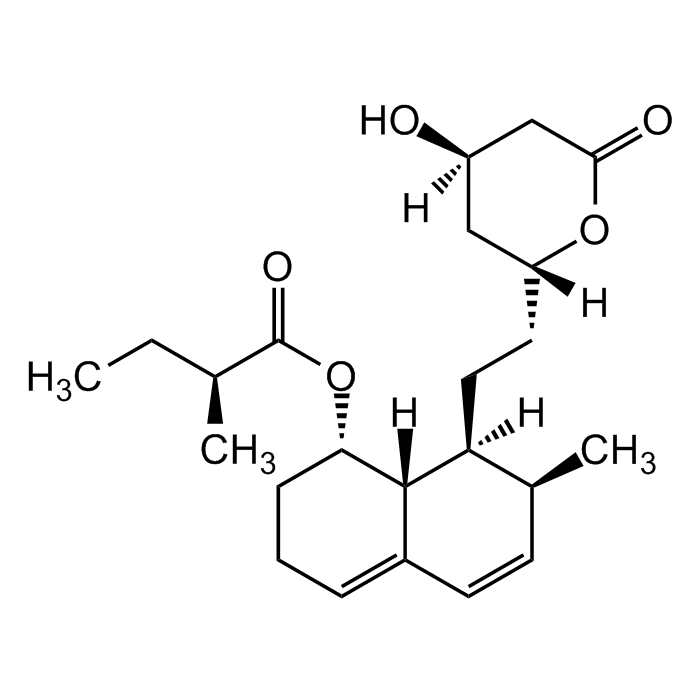
Mevastatin
| Product Details | |
|---|---|
| Synonyms | Compactin; 6-Demethylmevinolin; CS-500; ML-236B; L-637,312; NSC 281245; Statin I |
| Product Type | Chemical |
| Properties | |
| Formula | C23H34O5 |
| MW | 390.51 |
| CAS | 73573-88-3 |
| RTECS | EK7907100 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | White to light yellow powder. |
| Solubility | Soluble in DMSO or ethanol (20mg/ml). Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AJLFOPYRIVGYMJ-INTXDZFKSA-N |
| Smiles | [H][C@@]12C(C=C[C@H](C)[C@]2([H])CC[C@@]3([H])OC(C[C@@](O)([H])C3)=O)=CCC[C@@H]1OC([C@@H](C)CC)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Mevastatin is an antibiotic that is a competitive HMG-CoA reductase inhibitor, thus suppressing Ras farnesylation. Mevastatin decreases cholesterol biosynthesis in vitro and in vivo. It induces apoptosis, arrests cancer cells in G1 phase and downregulates cdk 2, 4, and 6, cyclin D1 and E1, p21 and p27. Mevastatin also suppresses TNF-induced NF-κB activation and potentiates apoptosis in human myeloid leukemia cells. Mevastatin induced neurite outgrowth of neuroblastoma cells via activation of EGFR, ERK1/2 and Akt/PBK but also exerts a neurotoxic effect in cultured neurons in a heme-independent manner. Mevastatin also shows antileishmanial and antifungal activity. Has also anti-inflammatory effects potentially via up-regulation of heme oxygenase-1 (HO-1).
[1] A. Endo, et al.; FEBS Lett. 72, 323 (1976) | [2] R. Fears, et al.; Atherosclerosis 35, 439 (1980) | [3] K. Keyomarsi, et al.; Cancer Res. 51, 3602 (1991) | [4] A. Endo; J. Lipid Res. 33, 1569 (1992) | [5] S. Amin-Hanjani, et al; Stroke 32, 980 (2001) | [6] A. Wachtershauser, et al.; Carcinogenesis 22, 1061 (2001) | [7] K.S. Ahn, et al.; Biochem. Pharmacol. 75, 907 (2008) | [8] M.E. Evangelopoulos, et al.; J. Neurosci. Res. 87, 2138 (2009) | [9] M. Kannan, et al.; Neurobiol. Aging 31, 1543 (2010) | [10] N. Dinesh, et al.; Parasitol. Res. 114, 3873 (2015) | [11] S. Javed, et at.; Curr. Pharm. Biotechnol. 17, 291 (2016) | [12] Z. Lin, et al.; Oncotarget 8, 17833 (2017) | [13] C.-C. Lin, et al.; J. Clin. Med. 9, 226 (2020)





