Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
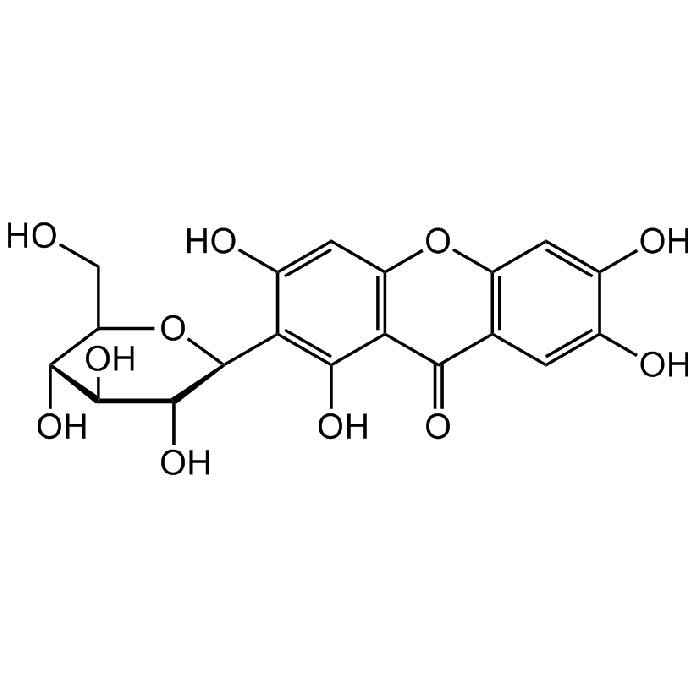
Mangiferin
| Product Details | |
|---|---|
| Synonyms | MGF; 1,3,6,7-Tetrahydroxyxanthone C2-β-D-glucoside; NSC 248870 |
| Product Type | Chemical |
| Properties | |
| Formula |
C19H18O11 |
| MW | 422.34 |
| CAS | 4773-96-0 |
| RTECS | OP1927800 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Light yellow powder. |
| Solubility | Soluble in DMSO (5mg/ml) or DMF (2mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AEDDIBAIWPIIBD-ZJKJAXBQSA-N |
| Smiles | OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](C2=C(O)C(C(C(C=C(O)C(O)=C3)=C3O4)=O)=C4C=C2O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Mangiferin is a xanthone glucoside that has been found in M. indica with diverse biological activities, including analgesic, antidiabetic, antisclerotic, antimicrobial and antiviral, cardio-, hepato-, and neuroprotective, anti-inflammatory, immunomodulatory, anticancer, as well as radioprotective against X-ray, gamma and UV radiation effects. It is also used in cosmetics, due to antioxidant and UV-protecting properties. The anti-inflammatroy activity is through inhibiting NF-κ and NLRP3 signaling pathways.
(1) F. Gold-Smith, et al.; Nutrients 8, E396 (2016) (Review) | (2) S. Saha, et al.; Biofactors 42, 459 (2016) (Review) | (3) A.J. Nunez Selles, et al.; Biofactors 42, 475 (2016) (Review) | (4) E.V. Fomenko & Y. Chi; Biofactors 42, 492 (2016) (Review) | (5) M. Imran, et al.; Lipids Health Dis. 16, 84 (2017) (Review) | (6) S. Du, et al.; Mol. Med. Rep. 18, 4775 (2018) (Review) | (7) F. Quadri, et al.; Expert Opin. Ther. Pat. 29, 463 (2019) (Review) | (8) S.T. Feng, et al.; Pharmacol. Res. 146, 104336 (2019) (Review)





