Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
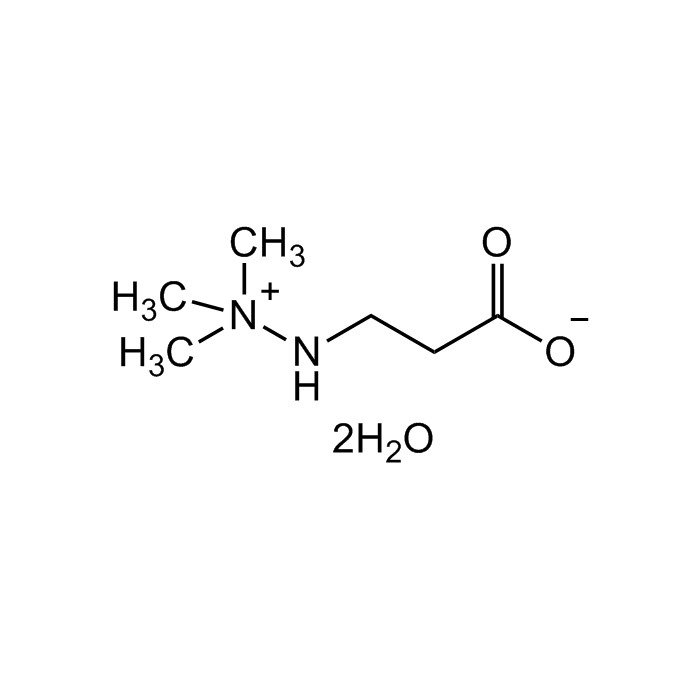
Mildronate dihydrate
| Product Details | |
|---|---|
| Synonyms | Meldonium; MET 88; Quaterin; THP |
| Product Type | Chemical |
| Properties | |
| Formula |
C6H14N2O2 . 2H2O |
| MW | 182.22 |
| CAS | 86426-17-7 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (20mg/ml) or ethanol (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PVBQYTCFVWZSJK-UHFFFAOYSA-N |
| Smiles | [O-]C(CCN[N+](C)(C)C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Mildronate is a structural analog of γ-butyrobetaine (γBB), an intermediate in the biosynthesis of carnitine and a modulator of energy metabolism. It blocks carnitine synthesis by inhibiting γBB hydroxylase (BBOX) and, less potently, carnitine acetyltransferase (CrAT). Through these actions, mildronate reduces the levels of free carnitine and long chain acyl carnitine. This leads to suppressed fatty acid metabolism and mitochondrial uncoupling during oxidative conditions, resulting in cardioprotective, antidiabetic and neuroprotective effects. Mildronate also improves cognition and reduces amyloid-β pathology in a mouse model of Alzheimer’s disease.
(1) B.Z. Simkhovich, et al.; Biochem. Pharmacol. 37, 195 (1988) | (2) M. Dambrova, et al.; Trends Cardiovasc. Med. 12, 275 (2002) (Review) | (3) E. Liepinsh, et al.; J. Cardiovasc. Pharmacol. 48, 314 (2006) | (4) R. Vilskersts, et al.; Pharmacology 83, 287 (2009) | (5) K. Jaudzems, et al.; J. Enzyme Inhib. Med. Chem. 24, 1269 (2009) | (6) J. Pupure, et al.; Neurosci. Lett. 470, 100 (2010) | (7) V.Z. Klusa, et al.; Int. J. Mol. Sci. 11, 4465 (2010) | (8) I.K. Leung, et al.; Chem. Biol. 17, 1316 (2010) | (9) E. Liepinsh, et al.; Eur. J. Pharmacol. 658, 277 (2011) | (10) K. Tars, et al.; J. Med. Chem. 57, 2213 (2014) | (11) U. Beitnere, et al.; J. Neurosci. Res. 92, 338 (2014) | (12) M. Makrecka, et al.; Eur. J. Pharmacol. 723, 55 (2014)





