Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
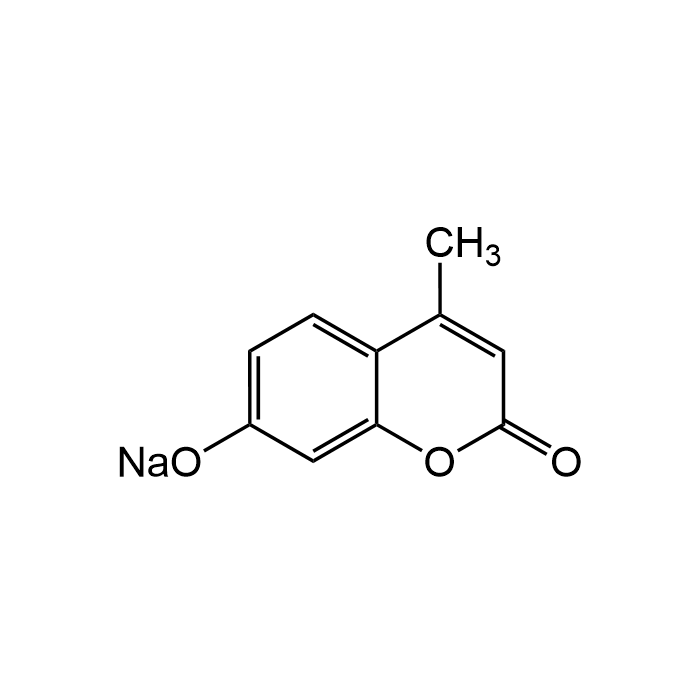
4-Methylumbelliferone sodium salt
| Product Details | |
|---|---|
| Synonyms | 4-MU sodium salt; β-Methylumbelliferone sodium salt; 7-Hydroxy-4-methylcoumarin sodium salt; Mendiaxon sodium salt; Cantabilin sodium salt |
| Product Type | Chemical |
| Properties | |
| Formula | C10H7O3 . Na |
| MW | 198.15 |
| CAS | 5980-33-6 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (NMR) |
| Appearance | Solid. |
| Solubility | Soluble in water (50 mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | JGMQHDNPUCPRQE-UHFFFAOYSA-M |
| Smiles | CC(C1=CC=C(O[Na])C=C1O2)=CC2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
4-Methylumbelliferone sodium salt is a salt form of 4-Methylumbelliferone (4-MU), which is a selective inhibitor of hyaluronan (HA) by depletion of UDP-glucuronic acid, the common substrate for HA synthesis and downregulation of hyaluronan synthase 2 and 3, MMP2 and MMP9. Since hyaluronan (HA) is a major component of the extracellular matrix that is synthesized in excess in cancer tissues, 4-MU has been shown to have anti-cancer activity and is involved in angiogenesis, invasion and metastasis of cancer. Fluorescent pH indicator, that is highly fluorescent in alkaline solution. Exhibits a blue fluorescence at pH7. The fluorescence intensity is pH dependent and increases to a maximum at pH10 (plateau above pH10). The fluorescence at pH10.3 is approximately 100 times as intense as at pH7. Spectral data: λex=360nm; λem=448nm; pH > 9. 4-Methylumbelliferone is an indicator also used in the determination of nitric acid and in 4-MU-O-substrates for the kinetic investigation of enzyme activity.
(1) R.F. Chen; Anal. Lett. 1, 423 (1968) | (2) T. Nakamura, et al.; Biochem. Mol. Biol. Int. 43, 263 (1997) | (3) I. Jones, et al.; Mol. Gen. Metab. 108, S51 (2003) | (4) I. Kakizaki, et al.; J. Biol. Chem. 279, 33281 (2004) | (5) S. Yoshihara, et al.; FEBS Lett. 579, 2722 (2005) | (6) R. Nakamura, et al.; Cell Biol. Int. 31, 1022 (2007) | (7) D. Vigetti, et al.; Glycobiology 19, 537 (2009) | (8) A. Kultti, et al.; Exp. Cell Res. 315, 1914 (2009) | (9) H. Urakawa, et al.; Int. J. Cancer 130, 454 (2012) | (10) J.A. Garcia-Vilas, et al.; J. Agric. Food Chem. 61, 4063 (2013) | (11) H. Zhi, et al.; J. Spectrosc. 2013, ID 147128 (2013) | (12) N. Nagy, et al.; Front. Immunol. 6, 123 (2015) (Review) | (13) H. Ban, et al.; Anticancer Res. 35, 5231 (2015)





