Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
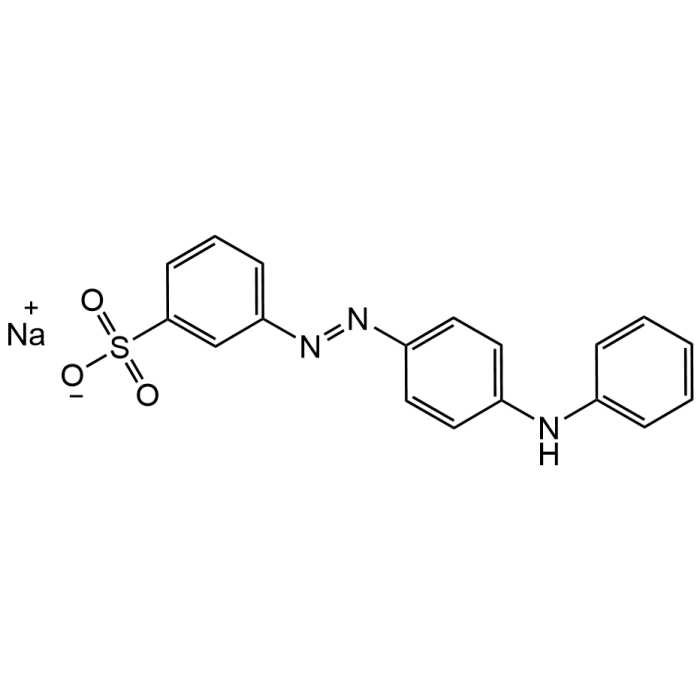
Acid Yellow 36
As low as
103
CHF
CHF 103.00
In stock
Only %1 left
CDX-M0849-G01010 gCHF 103.00
| Product Details | |
|---|---|
| Synonyms | C.I. Acid Yellow 36 monosodium salt; C.I. 13065; Acid Leather Yellow R; Metanile Yellow O; Sodium m-sulfonate-p-phenylazodiphenylamine; Yellow No. 406; 3-(4-Anilinophenylazo)benzenesulfonic acid sodium salt |
| Product Type | Chemical |
| Properties | |
| Formula | C18H14N3NaO3S |
| MW | 375.38 |
| CAS | 587-98-4 |
| RTECS | DB7329500 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow to beige to brown powder or crystals. |
| Solubility | Soluble in water, alohol or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PBAMGXBJWKWSID-UHFFFAOYSA-N |
| Smiles | O=S([O-])(C1=CC(/N=N/C2=CC=C(NC3=CC=CC=C3)C=C2)=CC=C1)=O.[Na+] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Acid Yellow 36 is an industrial azo dye used in textiles, leather, and paper, but should not be used in foods or cosmetics due to toxicity concerns. Metanil yellow changes its color from red at 1.2 pH to yellow at pH 2.5 and has been used as a pH indicator. Metanil yellow is used as a counterstain for microscopy sections, for staining of leukocyte granules, or as a counterstain in PAS (periodic acid-Schiff)/iron-hematoxylin stained sections to increase contrast. Spectral Data: λmax ~415 nm.
Product References
(1) E.B. Simmel, et al.; Stain Technol. 26, 25 (1951) | (2) N. Roman, et al.; Stain Technol. 42, 199 (1967) | (3) M.M. Everett & W.A. Miller; Stain Technol. 53, 315 (1978) | (4) O.M. Prasad & P.B. Rastogi; Toxicol. Lett. 16, 103 (1983) | (5) M. Opas & V.I. Kalnins; J. Microsc. 133, 291 (1984) | (6) I. Quintero-Hunter, et al.; Biotech. Histochem. 66, 169 (1991) | (7) J.A. Kiernan; Biotech. Histochem. 76, 261 (2001) | (8) R.W. Sabnis; Handbook of Acid-Base Indicators (2007) | (9) X. Liu, et al.; Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 28, 1315 (2011)





