Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
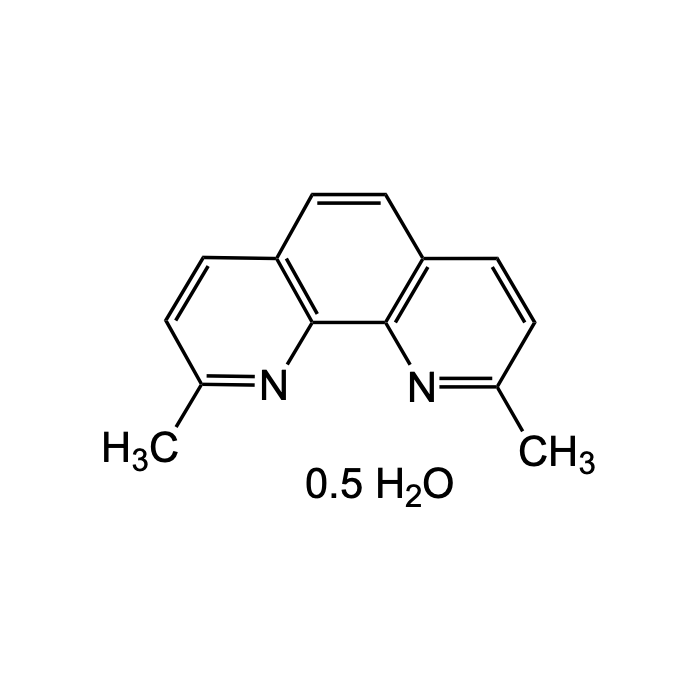
Neocuproine hemihydrate
| Product Details | |
|---|---|
| Synonyms | 2,9-Dimethyl-1,10-phenanthroline; DMPHEN; NSC 4280; VUF 7738 |
| Product Type | Chemical |
| Properties | |
| Formula |
C14H12N2 . 0.5H2O |
| MW | 217.27 |
| CAS | 484-11-7 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥99% (Assay) |
| Appearance | White to light yellow powder. |
| Solubility | Soluble in methanol (50mg/ml), ethanol (10mg/ml) or DMSO (25mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | IEBXFSLFDFHSRD-UHFFFAOYSA-N |
| Smiles | CC1=NC2=C(C=C1)C=CC3=C2N=C(C=C3)C.CC1=NC2=C(C=C1)C=CC3=C2N=C(C=C3)C.O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Neocuproine is a complexing reagent, known for its ability to form stable complexes with metal ions, particularly CU2+ ions. This property makes it valuable in various analytical and research applications, including spectrophotometry and electrochemistry. In the presence of CU2+ ions, neocuproine forms a brightly colored complex that can be used for the quantitative determination of copper in solution. The Neocuproine-CU2+ complex exists in a 2:1 ratio with a maximum absorption at 454 nm. Neocuproine is used in complex with Cu2+ as chromogenic oxidant in the total antioxidant capacity assay (CUPRAC method). In addition to its analytical uses, neocuproine and its derivatives have been employed in the field of coordination chemistry to study and synthesize metal complexes. These complexes often have unique properties and are of interest for their potential applications in catalysis, material science, and other areas of chemical research. This compound is also widely used in some biomedical fields, such as in the study of copper metabolism disorders and neurodegenerative diseases and neocuproine-CU2+ complexes have shown biological properties, such as antitumor activity.
(1) A. Mohindru, et al.; Biochem. Pharmacol. 32, 3627 (1983) | (2) H.H. Al-Sa?doni, et al.; Br. J. Pharmacol. 121, 1047 (1997) | (3) J.G. De Man, et al.; Eur. J. Pharmacol. 381, 151 (1999) | (4) R. Apak, et al.; Free Radic. Res. 39, 949 (2005) | (5) A.A. Gouda & A.S. Amin; Arabian J. Chem. 3, 159 (2010) | (6) O.V. Patel, et al.; Biometals 26, 415 (2013) | (7) K.A. Jesse, et al.; Inorg. Chem. 58, 9057 (2019)





