Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
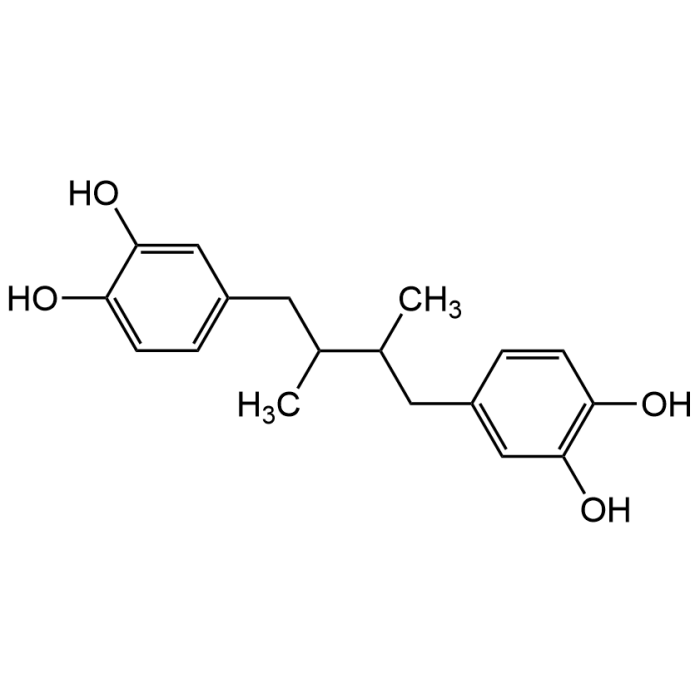
Nordihydroguaiaretic acid
As low as
64
CHF
CHF 64.00
In stock
Only %1 left
CDX-N0178-G0011 gCHF 64.00
CDX-N0178-G0055 gCHF 258.00
| Product Details | |
|---|---|
| Synonyms | NDGA; Dihydronorguaiaretic acid; NSC 4291; 1,4-Bis(3,4-dihydroxyphenyl)-2,3-dimethylbutane |
| Product Type | Chemical |
| Properties | |
| Formula | C18H22O4 |
| MW | 302.37 |
| CAS | 500-38-9 |
| RTECS | UX1750000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to light beige powder or crystals. |
| Solubility | Soluble in DMSO or ethanol (both 100mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HCZKYJDFEPMADG-UHFFFAOYSA-N |
| Smiles | C(C(C(CC1=CC(O)=C(O)C=C1)C)C)C2=CC(O)=C(O)C=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Nordihydroguaiaretic acid (NDGA) is a potent free radical scavenger, protecting cells and tissues from oxidative stress. It is a non-selective lipoxygenase (LO) inhibitor which blocks cysteinyl leukotriene (CysLT) synthesis. It is an inhibitor of the HER2 and IGF-1 receptor tyrosine kinases. NDGA inhibits p300 and activates autophagy. This compound shows antioxidant, antimutagenic, anticancer, antiviral and anti-inflammatory characteristics mediated through multiple signaling pathways.
Product References
(1) T. Nakadate, et al.; Gan 73, 841 (1982) | (2) S.J. Korn & R. Horn; Mol. Pharmacol. 38, 524 (1990) | (3) Z.Y. Wang, et al.; Mutat. Res. 261, 153 (1991) | (4) M. Pavani, et al.; Biochem. Pharmacol. 48, 1935 (1994) | (5) R.W. McDonald, et al.; Anticancer Drug Des. 16, 261 (2001) | (6) T. Seufferlein, et al.; Br. J. Cancer. 86, 1188 (2002) | (7) C.H. Lee, et al.; Exp. Cell Res. 289, 335 (2003) | (8) E. Floriano-Sanchez, et al.; Free Radic. Res. 40, 523 (2006) | (9) M. Zavodovskaya, et al.; J. Cell Biochem. 103, 624 (2008) | (10) Q. Chen; Curr. Top. Med. Chem. 9, 1636 (2009) (Review) | (11) J.M. Lu, et al.; Med. Sci. Monit. 16, RA93 (2010) (Review) | (12) T. Tezil, et al.; NPJ Aging Mech. Dis. 5, 7 (2019) | (13) G. Manda, et al.; Front. Pharmacol. 11, 151 (2020) | (14) F. Martinez, et al.; Phytomedicine 106, 154424 (2022)





