Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
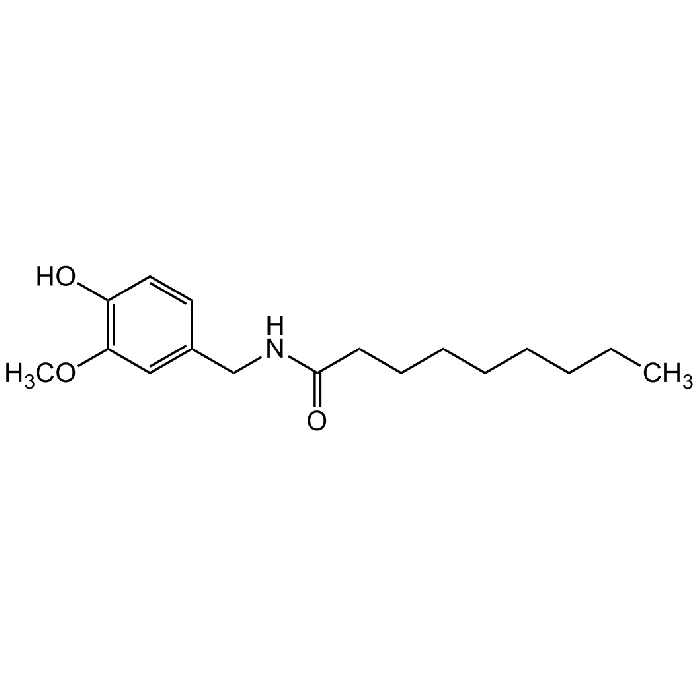
N-Vanillylnonanamide
| Product Details | |
|---|---|
| Synonyms | Nonivamide; N-(4-Hydroxy-3-methoxybenzyl)nonanamide; Nonanoic acid vanillylamide; Nonylvanylamide; NSC 172795; Pelargonic Acid Vanillylamide; Pseudocapsaicin; N-Vanillylpelargonamide |
| Product Type | Chemical |
| Properties | |
| Formula |
C17H27NO3 |
| MW | 293.41 |
| CAS | 2444-46-4 |
| RTECS | RA5955000 |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (10mg/ml), DMF (10mg/ml), ethanol (30mg/ml) or methanol (100mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | RGOVYLWUIBMPGK-UHFFFAOYSA-N |
| Smiles | OC1=C(OC)C=C(CNC(CCCCCCCC)=O)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Nonivamide is a transient receptor potential vanilloid type 1 (TRPV1) agonist and an analog of capsaicin, with anti-inflammatory, antiobesity and antifouling properties. Nonivamide has anti-inflammatory potential by inhibiting LPS-induced activation of the MAPK pathway. Nonivamide enhanced hyperthermia-induced apoptosis via a mitochondrial-caspase dependent pathway, and might be useful as cancer treatment enhancer. Nonivamide has also shown to be effective antiobesity agent by reducing energy intake, enhancing energy metabolism, decreasing serum triacylglycerol content and inhibiting adipogenesis via activation of the transient receptor potential cation channel subfamily V member 1 (TRPV1). Formulations containing nonivamide have been used in pepper spray weaponry for riot control.
(1) G. Skofitsch, et al. Arzneimittelforschung 34, 154 (1984) | (2) H.L. Constant & G.A. Cordell; J. Nat. Prod. 59, 425 (1996) | (3) C.A. Reilly, et al.; J. Forensic Sci. 46, 502 (2001) | (4) F. Villa, e al.; Biotechnol. Lett. 31, 1407 (2009) | (5) K.C. Thomas, et al.; J. Pharmacol. Exp. Ther. 337, 400 (2011) | (6) B. Rohm, et al.; Mol. Nutr. Food Res. 57, 2008 (2013) | (7) B. Rohm, et al.; J. Cell Biochem. 116, 1153 (2015) | (8) J. Walker, et al.; Mol. Nutr. Food Res. 61, 1600474 (2017) | (9) C.M. Hochkogler, et al.; Mol. Nutr. Food Res. 61, 1600731 (2017) | (10) L. Sun, et al.; Free Radic. Biol. Med. 120, 147 (2018)





