Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
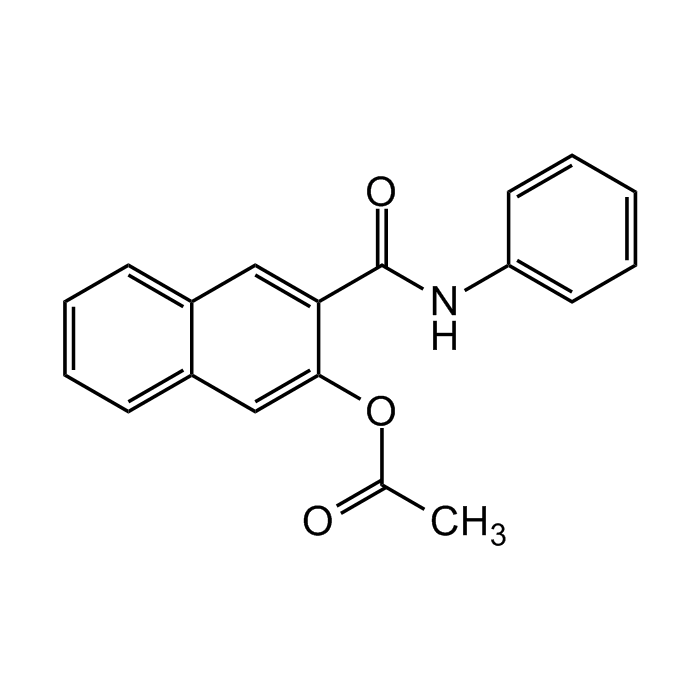
Naphthol AS acetate
| Product Details | |
|---|---|
| Synonyms | 3-Acetoxy-2-naphthoic acid anilide |
| Product Type | Chemical |
| Properties | |
| Formula | C19H15NO3 |
| MW | 305.33 |
| CAS | 1163-67-3 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | CVJGNNYDVQYHEO-UHFFFAOYSA-N |
| Smiles | O=C(NC1=CC=CC=C1)C2=CC3=CC=CC=C3C=C2OC(C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep under inert gas. Very hygroscopic. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Naphthol AS acetate is a fluorogenic substrate for unspecified esterases. After cleavage of the fluorigenic substrate by esterases the free Naphthol AS can couple with diazonium salts to form different types of fluorescent azo dyes. Human and bovine serum albumin are active against Naphthol AS acetate, resulting in a fluorescence excited at 320nm and monitored at 500nm. Can also be used as a bulding block or intermediate for organic synthesis.
(1) A.H. Baillie; J. Micr. Sci. 105, 247 (1964) | (2) V.K. Hopsu, et al.; J. Histochem. Cytochem. 13, 117 (1965) | (3) M.T. Rakhawy; Acta Anatomica 95, 572 (1976) | (4) M. Tavassoli; Scand. J. Haematol. 20, 330 (1978) | (5) Y. Eishi, et al.; Bull. Tokyo Med. Dent. Univ. 29, 47 (1982) | (6) R.F. Chen & C.H. Scott; Anal. Lett. 17, 857 (1984) | (7) U. Waspi, et al.; Eur. J. Biochem. 254, 32 (1998)





