Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
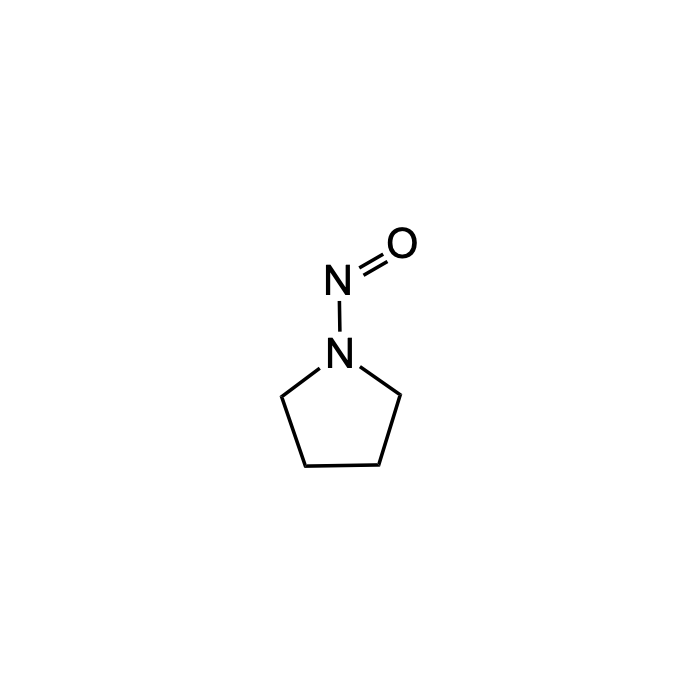
N-Nitrosopyrrolidine (NPYR)
| Product Details | |
|---|---|
| Synonyms | NPYR; NSC 18797; BRN 0107615 |
| Product Type | Chemical |
| Properties | |
| Formula | C4H8N2O |
| MW | 100.12 |
| CAS | 930-55-2 |
| RTECS | UY1575000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Pale yellow to yellow brown liquid. |
| Solubility | Soluble in chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | WNYADZVDBIBLJJ-UHFFFAOYSA-N |
| Smiles | C1CCN(C1)N=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
N-Nitrosopyrrolidine (NPYR) is a nitrosamine and is known to be a potent carcinogen associated with cancer development in animals and humans. NPYR can damage DNA and cause mutations in genes that control cell growth and division. It reacts with DNA in vitro to form genotoxic activity, which may lead to cell death or mutagenesis. NPYR is formed in various industrial processes, including the synthesis of certain chemicals where it is used as an organic solvent, pharmaceutical intermediate, and plasticizer. Functions as an analytical reagent for environmental monitoring. This compound can be used as analytical reference material. NPYR could be used as a tumor initiator, as a reagent for preparing carcinogenic animal diseases model. NPYR has also neurological effects, inhibiting NMDA receptors.
(1) W. Lijinsky & H.W. Taylor; Cancer Res. 36, 1988 (1976) | (2) Y.Y. Fong & W.C. Chan; Food Cosmet. Toxicol. 15, 143 (1977) | (3) S.S. Hecht, et al.; Cancer Res. 38, 215 (1978) | (4) J.I. Gray & M.E. Collins; J. Food Prot. 41, 36 (1978) | (5) M. Habs, et al.; Int. J. Cancer 26, 47 (1980) | (6) E.J. Hunt & R.C. Shank; BBRC 104, 1343 (1982) | (7) O. Manzoni, et al.; Neuron 8, 653 (1992) | (8) S. Krishnan, et al.; Chem. Commun. 17, 1713 (2007) | (9) Y. Li & S.S. Hecht; Int. J. Mol. Sci. 23, 4559 (2022) | (10) M. Bignami, et al.; Efsa J. 21, e07884 (2023)





