Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
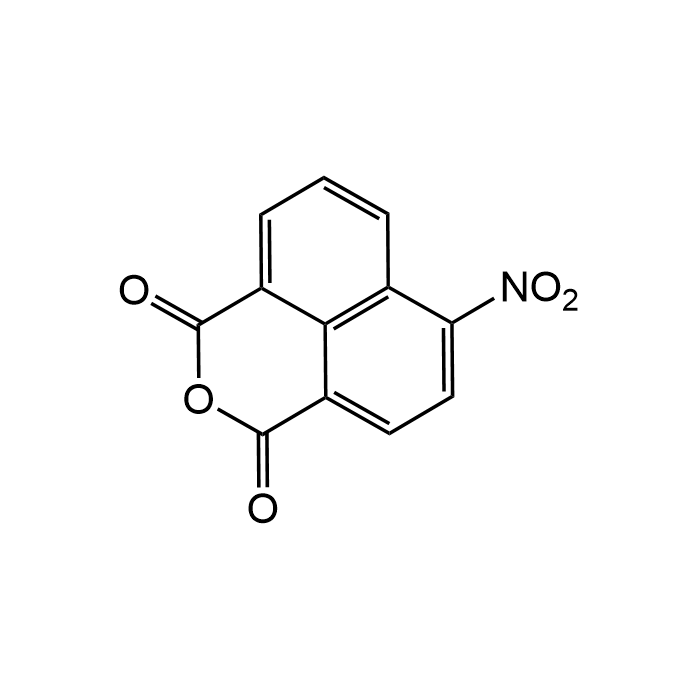
4-Nitro-1,8-naphthalic anhydride
As low as
193
CHF
CHF 193.00
In stock
Only %1 left
CDX-N0517-G0055 gCHF 193.00
| Product Details | |
|---|---|
| Synonyms | 4-NNA; 4-Nitronaphthalic anhydride; NSC 15356 |
| Product Type | Chemical |
| Properties | |
| Formula | C12H5NO5 |
| MW | 243.17 |
| CAS | 6642-29-1 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Yellow to brown solid. |
| Solubility | Soluble in DMSO. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LKOZHLXUWUBRDK-UHFFFAOYSA-N |
| Smiles | O=C(C1=CC=C([N+]([O-])=O)C2=C1C3=CC=C2)OC3=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
4-Nitro-1,8-naphthalic anhydride is a versatile chemical intermediate and building block for the synthesis of various organic compounds. It is used synthesizing N-phenyl-amino-1,8-naphthalimide fluorescent chemosensors, especially for nitro-antibiotic detection at ppb levels, and serves as a key precursor in manufacturing amphiphilic naphthalimide dyes with strong color and fluorescence properties. It also finds use in constructing flavorably designed shape-memory polymers, thanks to its reactive anhydride ring. Additionally, it's been employed as the substrate for nitroreductase activity assays, allowing quantification of enzyme activity by chromatographic means. The specific absorbance wavelengths of 4-NNA (in methanol) are 230 and 341nm in the ultraviolet range.
Product References
(1) J. Xu, et al.; J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 847, 82 (2007) | (2) A.-F. Miller, et al.; Molecules 23, 211 (2018) | (3) D. Staneva, et al.; Sensors 20, 3501 (2020) | (4) A. Shanmughan, et al.; Res. Chem. 4, 100546 (2022)





