Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Omeprazole sulfide
| Product Details | |
|---|---|
| Synonyms | Omeprazole impurity C (PhEur); Pyrmetazol; Ufiprazole |
| Product Type | Chemical |
| Properties | |
| Formula |
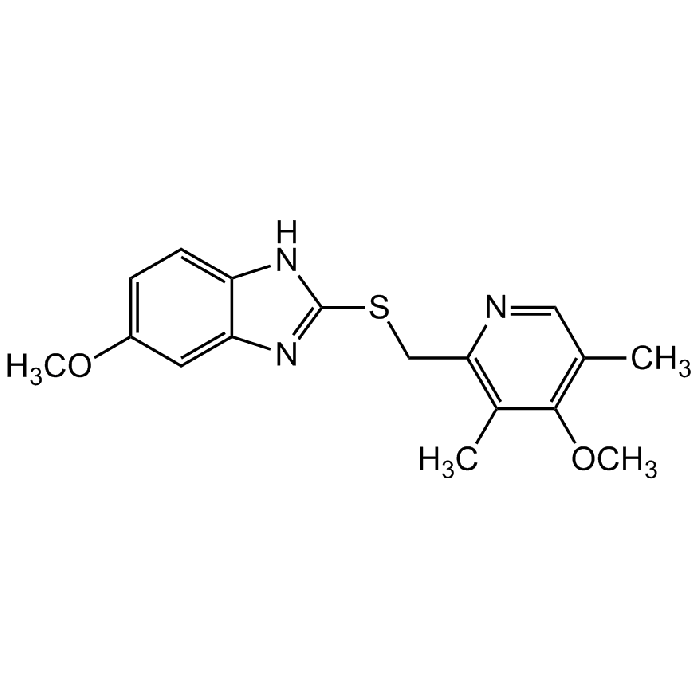
C17H19N3O2S |
| MW | 329.42 |
| CAS | 73590-85-9 |
| Purity Chemicals | ≥97% (NMR) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (10mg/ml), DMF (10mg/ml) or ethanol (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XURCIPRUUASYLR-UHFFFAOYSA-N |
| Smiles | CC(C(OC)=C(C)C=N1)=C1CSC2=NC3=CC(OC)=CC=C3N2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Omeprazole sulfide is an aryl hydrocarbon receptor (AhR) antagonist. Omeprazole sulfide is an intermediate used in the production of the gastric proton pump inhibitors, omeprazole and esomeprazole. Omeprazole sulfide is also a degradation product (Omeprazole Impurity C) of Omperazole, and it is reported to be a direct-acting inhibitor of cytochrome P450 2C19 in pooled human liver microsomes. Omeprazole is a selective and irreversible proton pump inhibitor. It suppresses stomach acid secretion by specific inhibition of the H+/K+-ATPase system found at the secretory surface of gastric parietal cells. Because this enzyme system is regarded as the acid (proton, or H+) pump within the gastric mucosa, omeprazole inhibits the final step of acid production. Omeprazole sulfide can be usd as reference material.
(1) S. Gerbal-Chaloin, et al.; Cell Signal. 18, 740 (2006) | (2) B.W. Ogilvie, e al.; Drug Metab. Dispos. 39, 2020 (2011) | (3) L. Olbe, et al.; Nat. Rev. Drug Discovery 2, 132 (2003) (Review)





