Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Osthole
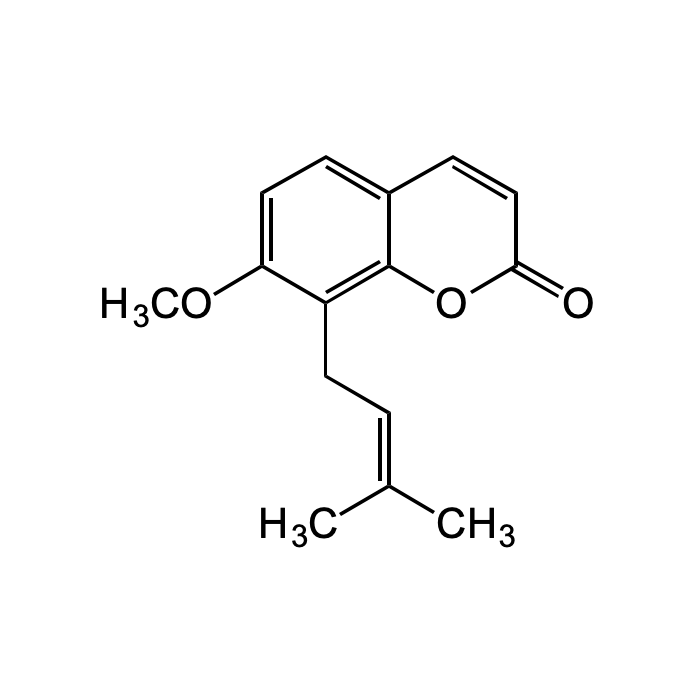
| Product Details | |
|---|---|
| Synonyms | 7-Methoxy-8-(3-methyl-2-butenyl)coumarin; 7-Methoxy-8-isopentenylcoumarin; NSC 31868 |
| Product Type | Chemical |
| Properties | |
| Formula | C15H16O3 |
| MW | 244.29 |
| CAS | 484-12-8 |
| RTECS | GN7700000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, DMF, ethanol (all 20mg/ml) or methanol (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | MBRLOUHOWLUMFF-UHFFFAOYSA-N |
| Smiles | O=C1OC2=C(C/C=C(C)/C)C(OC)=CC=C2C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Osthole is a natural coumarin that was first isolated from several medicinal plants, including C. monnieri and A. pubescens. Osthole exhibits immunomodulatory, antioxidative, and anti-inflammatory properties, by regulating the expression of TNF-α, NF-κB, TGF-β, cyclooxygenases, leukotrienes, nitric oxide, ERK and JNK. Shows also anti-cancer, chemopherapeutic, analgesic, neuroprotective, antipruritic, anti-leishmania and anti-lipedemic activities. It is reported to induce a nonspecific elevation of cAMP and cGMP levels through its ability to inhibit phosphodiesterases, which leads to a modulation of calcium channel signaling and vasorelaxant effects. Selectively inhibits TRPV3 channels in skin. Induces apoptosis and cell cycle arrest in cancer cells. Suppression of fatty acid synthase expression in HER2-overexpressing breast cancer cells indicates osthole as a promising agent for chemotherapy.
(1) R.L. Huang, et al.; Hepatology 24, 508 (1996) | (2) L. Wang, et al.; J. Biomed. Res. 29, 132 (2015) | (3) Z.R. Zhang, et al.; Evid.-Based Complem. Altern. Med. 2015, 919616 (2016) | (4) E.K. Kermani, et al.; Pharmacognosy Res. 8, S1 (2016) | (5) X.M. Xu, et al.; Oncol. Lett. 12, 3779 (2016) | (6) Y. Che, et al.; Oncol. Rep. 40, 737 (2018) | (7) X.Y. Sun, et al.; Mol. Pharmacol. 94, 1164 (2018) | (8) Y. Yao, et al.; Life Sci. 217, 16 (2019)





