Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
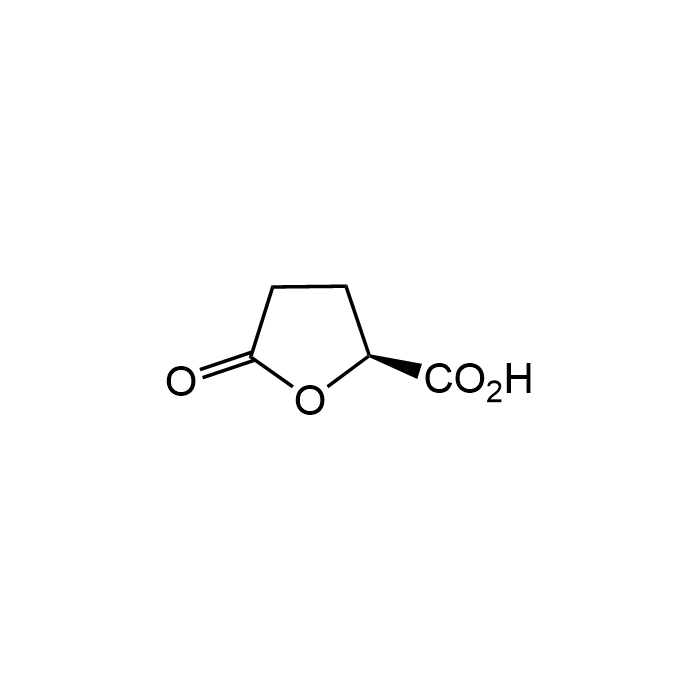
(S)-(+)-5-Oxo-2-tetrahydrofurancarboxylic acid
As low as
35
CHF
CHF 35.00
In stock
Only %1 left
CDX-O0227-G0011 gCHF 35.00
CDX-O0227-G0055 gCHF 135.00
| Product Details | |
|---|---|
| Synonyms | S-Hothf; (S)-γ-Carboxy-γ-butyrolactone; (S)-5-Oxotetrahydro-2-furancarboxylic acid |
| Product Type | Chemical |
| Properties | |
| Formula | C5H6O4 |
| MW | 130.1 |
| CAS | 21461-84-7 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥99% (GC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QVADRSWDTZDDGR-VKHMYHEASA-N |
| Smiles | O=C1CC[C@@H](C(O)=O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
(S)-(+)-5-Oxo-2-tetrahydrofurancarboxylic acid is a chiral lactone compound widely utilized in organic synthesis and medicinal chemistry. It serves as a versatile ligand for forming water-soluble silver(I) complexes, which exhibit notable antibacterial and antifungal properties. Additionally, this compound acts as a chiral resolving agent in the multi-step synthesis of phosphatidylinositol 3,5-bisphosphate, a crucial lipid involved in cellular signaling. Furthermore, it is employed in the preparation of municatacin analogs, which demonstrate cytotoxic activity against tumor cells, highlighting its potential in cancer research. Due to its unique structural features, (S)-(+)-5-Oxo-2-tetrahydrofurancarboxylic acid is an essential intermediate in the development of biologically active compounds and chiral catalysts. This compound has also been described as a reversible proline dehydrogenase (PRODH) inhibitor.
Product References
(1) B. Figadere; Tetrahedr. Lett. 32, 7539 (1991) | (2) A.M. Riley & B.V.L. Potter; Tetrahedr. Lett. 39, 6769 (1998) | (3) K. Nomiya, et al.; J. Chem. Soc. Dalton Trans. 8, 1343 (2000) | (4) G.K. Scott, et al.; Mol. Cancer Ther. 18, 1374 (2019) | (5) G. Pallag, et al.; Int. J. Mol. Sci. 23, 5111 (2022)





