Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
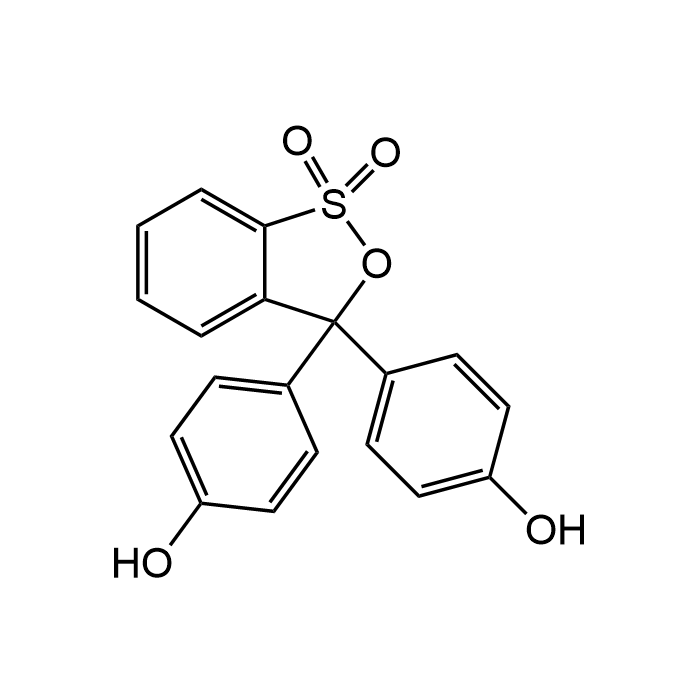
Phenol red
As low as
26
CHF
CHF 26.00
In stock
Only %1 left
CDX-P0025-G0011 gCHF 26.00
CDX-P0025-G01010 gCHF 71.00
CDX-P0025-G05050 gCHF 213.00
| Product Details | |
|---|---|
| Synonyms | Phenolsulfonphthalein; Sulfonphthal; PSP; NSC 10459; 3,3-Bis(p-hydroxyphenyl)-2,1-3H-benzoxathiole 1,1-dioxide |
| Product Type | Chemical |
| Properties | |
| Formula | C19H14O5S |
| MW | 354.38 |
| CAS | 143-74-8 |
| RTECS | SJ7490000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (Dye content) |
| Appearance | Powder. |
| Solubility | Soluble in DMSO. Slightly soluble in water, ethanol or methanol. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | BELBBZDIHDAJOR-UHFFFAOYSA-N |
| Smiles | O=S1(C2=CC=CC=C2C(C3=CC=C(O)C=C3)(C4=CC=C(O)C=C4)O1)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Phenol red belongs to the class of triphenylmethane dyes and is a widely used pH indicator and biological reagent, valued for its clear colorimetric response over a pH range of 6.8 to 8.4, transitioning from yellow to red. It is commonly employed in cell culture media to monitor pH changes during cell growth and metabolism, ensuring optimal conditions for biological assays. Widely used in culture media, phenol red identifies pH changes from neutral (red) to acidic (yellow) values. Typically a change in color from red to yellow indicates high rates of cellular death when used in tissue cultures. Beyond its role as a pH indicator, phenol red serves in analytical chemistry for titrations, as a visual marker in enzyme activity assays, and in the development of colorimetric sensors. In different protocols, it is used to detect hydrogen peroxide. Visual transition intervals: 1.2-3.0, brown-orange to yellow (1st transition range); 6.5-8.0, brownish-yellow to red-violet (2nd transition range).
Product References
(1) Y. Berthois, et al.; PNAS 83, 2496 (1986) | (2) K. Higaki, et al.; Pharm. Res. 5, 309 (1988) | (3) P. Yuan & D.R. Walt; J. Fluoresc. 2, 231 (1992) | (4) W. Yao & R.H. Byrne; Environ. Sci. Technol. 35, 1197 (2001) | (5) A. Sundarakrishnan, et al.; Acta Biomater. 42, 102 (2016) | (6) A. Morgan, et al.; Chem. Biol. Interact. 310, 108739 (2019) | (7) C. Sun, et al.; Spectrochim. Acta A Mol. Biomol. Spectrosc. 296, 122663 (2023) | (8) R.E. Foreman, et al.; Bioanalysis 16, 575 (2024) | (9) M. Vera, et al.; Free Radic. Biol. Med. 222, 397 (2024)





