Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
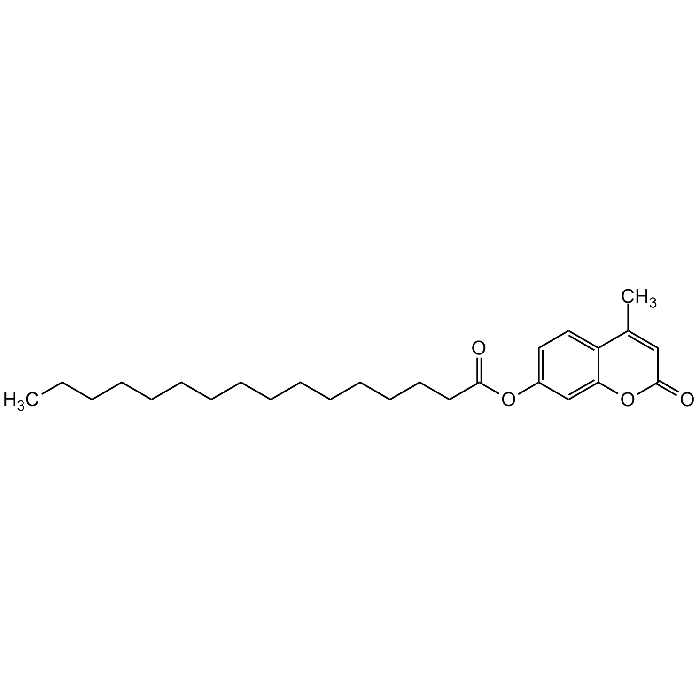
4-Methylumbelliferyl palmitate
| Product Details | |
|---|---|
| Synonyms | 4-MUP; 4-MU Palmitate; 4-Methyl-2-oxo-2H-chromen-7-yl palmitate; Palmitic acid 4-methylumbelliferyl ester |
| Product Type | Chemical |
| Properties | |
| Formula |
C26H38O4 |
| MW | 414.59 |
| CAS | 17695-48-6 |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | White to off-white crystalline powder. |
| Solubility | Soluble in DCM (10mg/ml), chloroform (20mg/ml) or DMF (10mg/m). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QKQVFFACWLFRRX-UHFFFAOYSA-N |
| Smiles | O=C1OC2=CC(OC(CCCCCCCCCCCCCCC)=O)=CC=C2C(C)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
4-Methylumbelliferyl palmitate (4-MUP) is a fluorogenic substrate for lysosomal acid lypase (LAL). 4-MUP is cleaved by LAL (or by other acid lipases) to release the fluorescent moiety 4-MU. Recent advances allow the assessment of LAL activity in very small blood volumes using 4-MUP. Cholesterol ester storage disease and Wolman disease are recessive autosomal disorders caused by a deficiency in lysosomal acid lipase (LAL), also known as cholesteryl ester hydrolase. Spectral data: 4-MU fluorescence is pH-dependent. λex 320nm (pH 1.97-6.72) and 360nm (pH 7.12-10.3); λem 445-455nM (increasing as pH decreases).
(1) G.J. Guy & J. Butterworth; Clin. Chim. Acta 84, 361 (1978) | (2) S. Kelly & R. Bakhru-Kishore; Clin. Chim. Acta 97, 239 (1979) | (3) T.G. Warner, et al.; Am. J. Hum. Gen. 32, 869 (1980) | (4) T.G. Warner, et al.; J. Biol. Chem. 256, 2952 (1981) | (5) M.W. Baillargeon & S.G. McCarthy; Lipids 26, 831 (1991) | (6) J. Hamilton, et al.; Clin. Chim. Acta 413, 1207 (2012) | (7) H. Zhi, et al.; J. Spectrosc. 1, 147128 (2013) | (8) T. Dairaku, et al.; Mol. Gen. Metab. 111, 193 (2014)





