Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
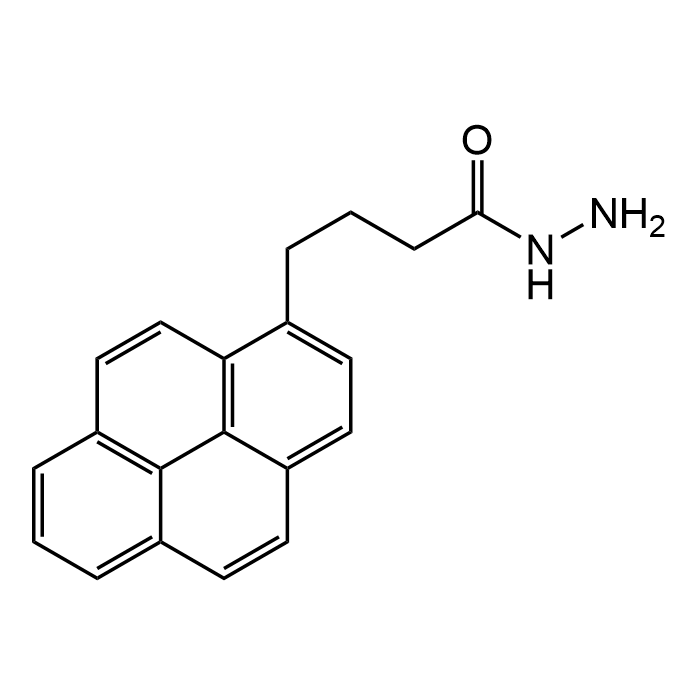
1-Pyrenebutanoic acid hydrazide
As low as
213
CHF
CHF 213.00
In stock
Only %1 left
CDX-P0049-M100100 mgCHF 213.00
CDX-P0049-M250250 mgCHF 412.00
CDX-P0049-G0011 gCHF 1’321.00
| Product Details | |
|---|---|
| Synonyms | PBH; 1-Pyrenebutyric hydrazide; Pyrenebutyrylhydrazine |
| Product Type | Chemical |
| Properties | |
| Formula | C20H18N2O |
| MW | 302.37 |
| CAS | 55486-13-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SOPPDVMSOKMZOC-UHFFFAOYSA-N |
| Smiles | O=C(NN)CCCC1=CC=C2C=CC3=CC=CC=4C=CC1=C2C34 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
1-Pyrenebutanoic acid hydrazide (PBH) is a fluorescent labeling reagent commonly used in biochemistry and molecular biology. Spectral Data: λex 340nm, λem 375nm (MeOH). The hydrazide group reacts with carbonyl groups (like aldehydes or ketones) to form hydrazones. This makes PBH useful for labeling oxidized sugars, glycoproteins, or carbonylated biomolecules. It can be used to modify surfaces (e.g., glass or nanoparticles) for biosensing applications. Also used in the derivatization of proteins, carbohydrates, or lipids that have been oxidized to expose reactive carbonyls. PBH is utilized in developing advanced optical materials, where its fluorescence properties can enhance the detection sensitivity of various biological and chemical systems.
Product References
(1) S.A. Reines & C.R. Cantor; Nucleic Acids Res. 1, 767 (1974) | (2) P. Koenig, et al.; Biopolymers 16, 2231 (1977) | (3) N. Shambaugh & E. Fujimori; Biopolymers 21, 79 (1982) | (4) D. Sugahara, et al.; Anal. Sci. 19, 167 (2003) | (5) H. Yoshida, et al.; Anal. Chim. Acta 534, 177 (2005) | (6) F.V Mansano, et al.; Anal. Chem. 82, 6775 (2010) | (7) F. Novio, et al.; Chem. Eur. J. 20, 15443 (2014) | (8) R.W. Sabnis; Handbook of Fluorescent Dyes and Probes 334 (2015)





