Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
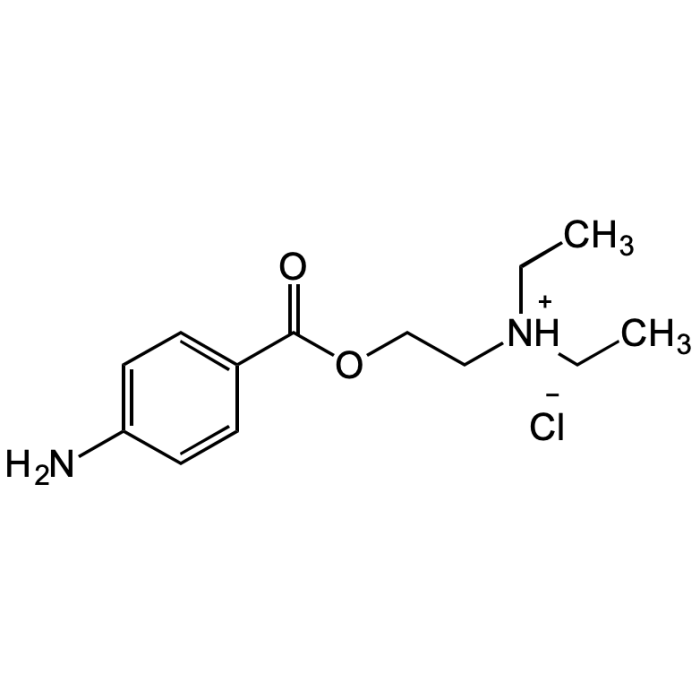
Procaine hydrochloride
| Product Details | |
|---|---|
| Synonyms | 4-Aminobenzoic acid 2-diethylaminoethyl ester; p-Aminobenzoic acid diethylaminoethyl ester hydrochloride; Novocaine hydrochloride |
| Product Type | Chemical |
| Properties | |
| Formula | C13H20N2O2 . HCl |
| MW | 272.77 |
| CAS | 51-05-8 |
| RTECS | DG2275000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water or ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HCBIBCJNVBAKAB-UHFFFAOYSA-N |
| Smiles | NC1=CC=C(C(OCC[NH+](CC)CC)=O)C=C1.[Cl-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Procaine is a sodium channel blocker, commonly used as an anesthetic agent and a variety of other inhibitiory effects. It is also useful as a painkiller to prevent or relieve pain for short periods, such as during dental procedures. In skeletal muscle, Procaine has been reported to inhibit calcium-induced calcium release (CICR) and to inhibit nicotinic acetylcholine receptors. It has been shown to be a DNA methylation inhibitor with anti-tumor activity. Inhibited also HERG channels. Can be used as an analytical reference compound, since procaine is used as an adulterant in illicit drugs.
(1) S. Epstein & N.W. Chilton; Oral Surg. Oral Med. Oral Pathol. 12, 93 (1959) | (2) R. Werdehausen, et al.; Br. J. Anaesth. 103, 711 (2009) | (3) Z. Gao, et al.; Oncol. Rep. 22, 1479 (2009) | (4) M. Endo; Physiol. Rev. 89, 1153 (2009) | (5) H. Wang, et al.; Eur. J. Pharmacol. 630, 29 (2010) | (6) R. Gal & F. Libersat; PLoS ONE 5, e10019 (2010) | (7) C. Cole, et al.; CUT (2010) | (8) N. Wang, et al.; J. Pharmacol. Sci. 123, 25 (2013) | (9) C. Li, et al.; Oncol. Res. 26, 209 (2018) | (10) Y.C. Li, et al.; J. Cell Biochem. 119, 2440 (2018)





