Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
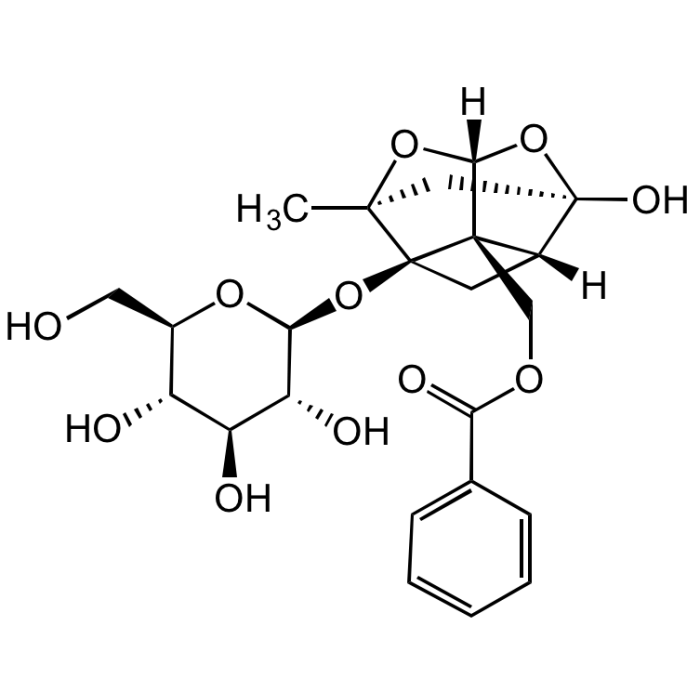
Paeoniflorin
| Product Details | |
|---|---|
| Synonyms | NSC 178886 |
| Product Type | Chemical |
| Properties | |
| Formula | C23H28O11 |
| MW | 480.46 |
| CAS | 23180-57-6 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, DMF, ethanol (all 20mg/ml) or water (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YKRGDOXKVOZESV-WRJNSLSBSA-N |
| Smiles | O[C@]12O[C@@]3([H])O[C@](C2)(C)[C@@]4([C@@]3(COC(C5=CC=CC=C5)=O)[C@@]1([H])C4)O[C@@H]([C@@H]([C@H]6O)O)O[C@H](CO)[C@H]6O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Paeoniflorin has diverse cellular actions, including modulating NMDA and TRPV1 receptors. It is reported to inhibit testosterone synthesis and stimulate aromatase activity. It also reduces inflammatory signaling by inhibiting p38 MAP kinase and blocks pancreatic cancer cell apoptosis by suppressing MMP-2/9 and ERK signaling. It has been described as an adenosine A1 receptor activator with neuroprotective and antidepressant effects, as well as hypoglycemic activity. It can activate adenosine A-1 receptors to increase the translocations of PKC and GLUT4, two major signals for glucose uptake, from cytosol to membrane of the white adipocytes in rats. Recently it also was described as a liver X receptor (LXR) agonist, that can act as a ligand-activated transcription factor to exhibit antihyperlipidemic and neuroprotective effects. In addition, this potential anticancer agent, has ben described as a EP2 receptor agonists, a heat-shock protein (HSP)-inducing compound, by activation of HSF1, or by interacting with NOTCH1 or mTOR/HIF-1 signaling pathways.
(1) J. Yamahara, et al.; J. Pharmacobiodyn. 5, 921 (1982) | (2) T. Takeuchi, et al.; Amer. J. Chin. Med. 19, 73 (1991) | (3) F.L. Hsu, et al.; Planta Med. 63, 323 (1997) | (4) C.W. Lai, et al.; Life Sci. 62, 1591 (1998) | (5) H.O. Yang, et al.; Fitoterapia 75, 45 (2004) | (6) D. Yan, et al.; Cell Stress Chaperones 9, 378 (2004) | (7) D.Z. Liu, et al.; Br. J. Pharmacol. 146, 604 (2005) | (8) H.Q. Liu, et al.; Br. J. Pharmacol. 148, 314 (2006) | (9) J.Y. Hung, et al.; Clin. Exp. Pharmacol. Physiol. 35, 141 (2008) | (10) H. Wu, et al.; Biomed. Pharmacother. 62, 659 (2008) | (11) J. Fu, et al.; Comp. Med. 59, 557 (2009) | (12) J. Huang, et al.; Int. Immunopharmacol. 10, 1279 (2010) | (13) Q.Q. Mao, et al.; Phytother. Res. 25, 681 (2011) | (14) S. Hu, et al.; Anticancer Drugs 24, 140 (2013) | (15) R.B. Guo, et al.; PLoS One 7, e49701 (2012) | (16) H.R. Lin; J. Asian Nat. Prod. Res. 15, 35 (2013) | (17) F.M. Qiu, et al.; Neurosci. Lett. 541, 209 (2013) | (18) W. Li, et al.; Mol. Med. Rep. 12, 2735 (2015) | (19) F. Han, et al.; Cell Biosci. 6, 37 (2016) | (20) S. Wang & W. Liu; Mol. Med. Rep. 14, 2143 (2016) | (21) R. Lu, et al.; Biomed. Pharmacother. 96, 137 (2017) | (22) J. Zhang, et al.; Mol. Med. Rep. 17, 1321 (2018) | (23) H. Wang, et al.; J. Cell Physiol. (Epub ahead of print) (2018) | (24) L.B. Jin, et al.; Mol. Med. Rep. 17, 5095 (2018)





