Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
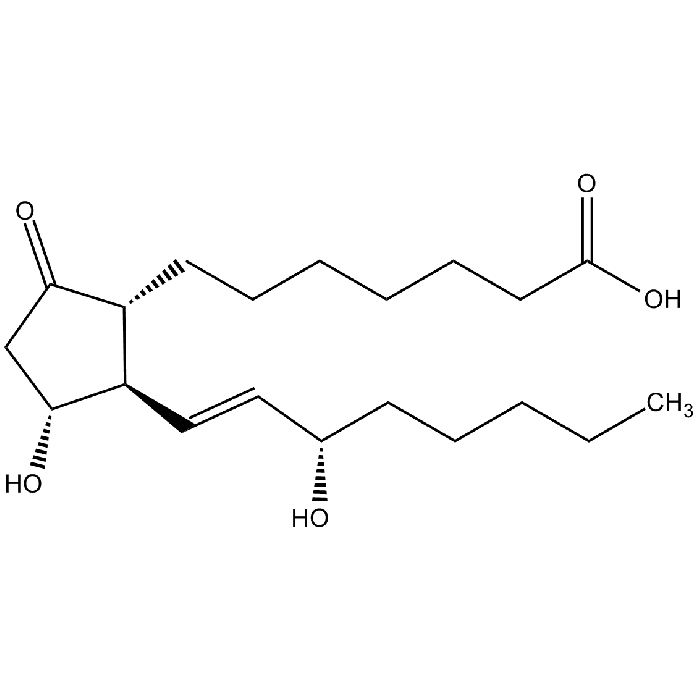
Prostaglandin E1
| Product Details | |
|---|---|
| Synonyms | (11α,13E,15S)-11,15-Dihydroxy-9-oxoprost-13-enoic acid; Alprostadil; PGE1 |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H34O5 |
| MW | 354.49 |
| CAS | 745-65-3 |
| RTECS | GY4569800 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (30mg/ml), DMF (50mg/ml) or ethanol (30mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | GMVPRGQOIOIIMI-DWKJAMRDSA-N |
| Smiles | O[C@@H]([C@H](/C=C/[C@@H](O)CCCCC)[C@H]1CCCCCCC(O)=O)CC1=O |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Prostaglandin E1 (PGE1) is an endogenous prostaglandin used as a medication. Its synthetic form, alprostadil, is a vasodilator and smooth muscle relaxant used for several different medical purposes. PGE1 is the theoretical cyclooxygenase metabolite of dihomo-γ-linolenic acid (DGLA), but it is virtually undetectable in the plasma of normal humans or other animals. Its pharmacology includes vasodilation, hypotension and anti-platelet activities. The IC50 of PGE1 for the inhibition of ADP-induced human platelet aggregation is 40 nM. The vasorelaxant and anti-hypertensive effects of PGE1 are used to treat male erectile dysfunction and to provide emergency vasodilation of the patent ductus arteriosus in infants whose cardiac anomalies require pulmonary shunting for survival.
(1) F. Okada, et al.; Prostaglandins 7, 99 (1974) | (2) P.M. Olley & F. Coceani; Ann. Rev. Med. 32, 375 (1981) | (3) S.J. Kirtland; Prostaglandins Leukot. Essent. Fatty Acids 32, 165 (1988) (Review) | (4) G. Kobzar, et al.; Proceed. Est. Acad. Sci. Chem. 40, 179 (1991) | (5) H. Padma-Nathan, et al.; New Engl. J. Med. 336, 1 (1997) | (6) W. Cawello, et al.; J. Urol. 158, 1403 (1997)





