Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
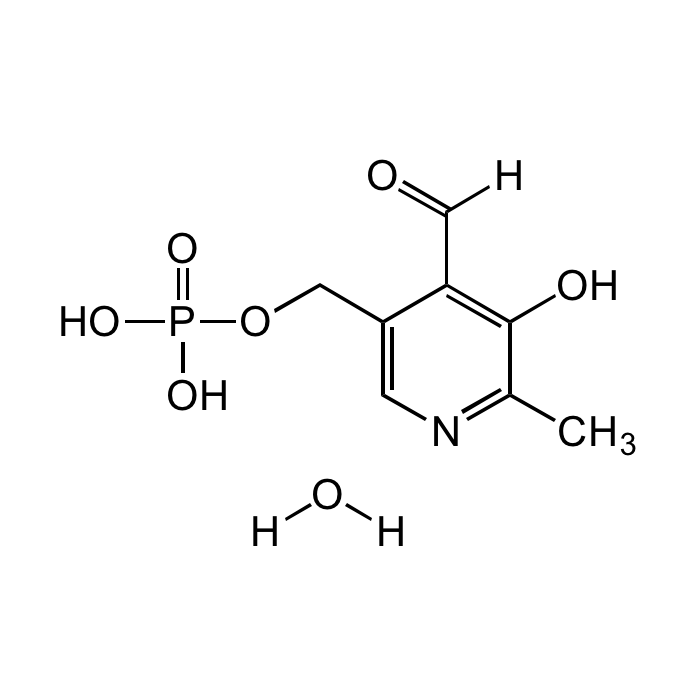
Pyridoxal 5'-phosphate monohydrate
| Product Details | |
|---|---|
| Synonyms | P5P; PLP; Codecarboxylase; 3-Hydroxy-2-methyl-5-([phosphonooxy]methyl)-4-pyridinecarboxaldehyde; Pyridoxal 5-phosphate; Vitamin B6 Metabolite |
| Product Type | Chemical |
| Properties | |
| Formula |
C8H10NO6P . H2O |
| MW | 265.16 |
| CAS | 41468-25-1 |
| RTECS | UV1208000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Slightly soluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | CEEQUQSGVRRXQI-UHFFFAOYSA-N |
| Smiles | O=C([H])C1=C(O)C(C)=NC=C1COP(O)(O)=O.[H]O[H] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
The enzyme cofactor Pyridoxal-5‘-phosphate monohydrate is a vitamin B6 metabolite that can modify lysyl and valyl residues in proteins. It acts as a coenzyme in transamination reactions by forming a Schiff-base linkage with lysine groups on aminotransferase. Also serves as a coenzyme in some decarboxylation and deamination reactions. It has the ability to inhibit purinergic receptors and intracellular influx of Ca2+. Pyridoxal-5‘-phosphate monohydrate can modify peptides and suppress their precursor ionization efficiency. Research shows that pyridoxal-5‘-phosphate-dependent enzymes can be inhibited by cycloserine.
(1) D.E. Kandzari, et al.; Am. J. Cardiol. 92, 660 (2003) | (2) D.W. Kim, et al.; J. Biochem. Mol. Biol. 38, 58 (2005) | (3) J.C. Tardif, et al.; J. Thorac. Cardiovasc. Surg. 133, 1604 (2007) | (4) M. Neuwirth, et al.; FEBS Lett. 583, 2179 (2009) | (5) E.S. Simon, et al.; Rapid Commun. Mass Spectrom 23, 3401 (2009) | (6) J. Lowther, et al.; Mol Biosyst. 6, 1682 (2010) | (7) J.L. Hedrick; Adv. Biochem. Psychopharmacol. 4, 23 (1972) | (8) B.I. Yang & D.E. Metzler; Methods Enzymol. 62, 528 (1979) | (9) G. Tunnicliff & T.T. Ngo; Cell Physiol. Biochem. 8, 117 (1998) | (10) P. Christen & P.K. Mehta; Chem. Rec. 1, 436 (2001) | (11) M.L. di Salvo, et al.; Front. Biosci 4, 897 (2012) | (12) L. Paul, et al.; Nutr. Rev. 71, 239 (2013)





