Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
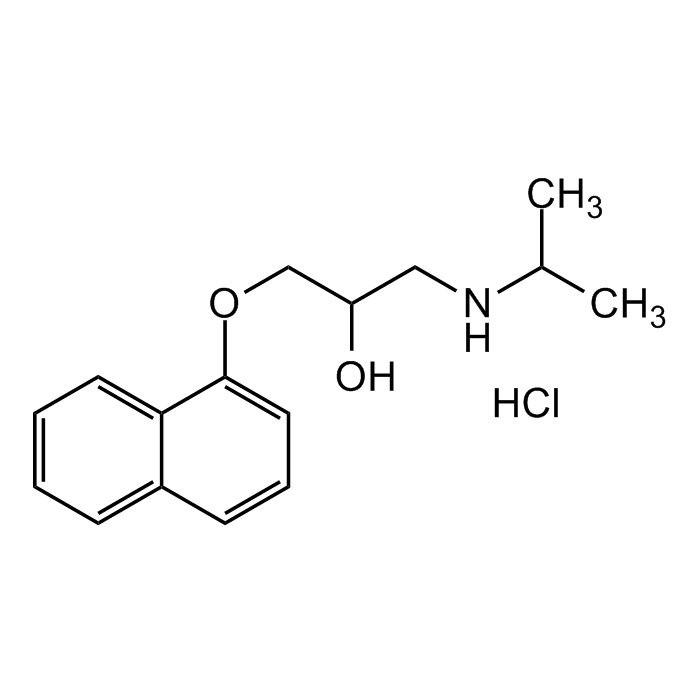
(±)-Propranolol hydrochloride
| Product Details | |
|---|---|
| Synonyms | (±)-1-Isopropylamino-3-(1-naphthyloxy)-2-propanol hydrochloride; DL-Propranolol hydrochloride; ICI 45520; NSC 91523 |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H21NO2 . HCl |
| MW | 295.8 |
| CAS | 318-98-9 |
| RTECS | UB7525000 |
| Purity Chemicals | ≥99% (TLC) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO (15mg/ml), ethanol (10mg/ml) or water (20mg/ml). Aqueous solutions are most stable at pH 3.0 and decompose rapidly at basic pH. Decomposition is accompanied by discoloration of the solution. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ZMRUPTIKESYGQW-UHFFFAOYSA-N |
| Smiles | OC(CNC(C)C)COC1=CC=CC2=CC=CC=C21.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep under inert gas. Very hygroscopic. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
(±)-Propranolol hydrochloride is a β-adrenergic receptor (β-AR) antagonist (Kds = 6.91, 0.832, and 117.49 nM for human β1-, β2- and β3-ARs, respectively). It also acts as a non-specific 5-HT1/5-HT2 serotonin receptor antagonist, reducing tranylcypromine/L-tryptophan-induced hyperactivity in rats. (±)-Propranolol hydrochloride is useful as an antihypertensive drug, cardiac depressant and also in the treatment of angina pectoris. It decreases the effect of stress and exercise on heart by reducing the rate of contraction and conduction of impulse. It is known to competitively block the action of catecholamines.
(1) J.D. Fitzgerald & S.R. O'Donnell; Br. J. Pharmacol. 45, 207 (1972) | (2) B. Basil, et al.; Br. J. Pharmacol. 50, 323 (1974) | (3) D.W. Costain & A.R. Green; Br. J. Pharmacol. 64, 193 (1978) | (4) S.E. Litwin, et al.; Br. J. Pharmacol. 127, 1671 (1999) | (5) J.G. Baker; Br. J. Pharmacol. 144, 317 (2005) | (6) M.J. Wagner, et al.; J. Exp. Pharmacol. 10, 51 (2018)





