Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
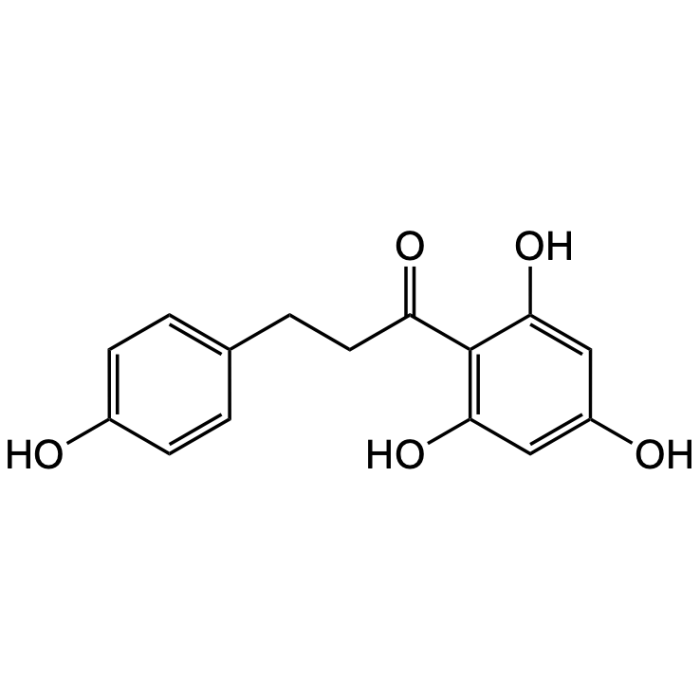
Phloretin
| Product Details | |
|---|---|
| Synonyms | 3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone |
| Product Type | Chemical |
| Properties | |
| Formula | C15H14O5 |
| MW | 274.27 |
| CAS | 60-82-2 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, ethanol or methanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VGEREEWJJVICBM-UHFFFAOYSA-N |
| Smiles | Oc1ccc(CCC(=O)c2c(O)cc(O)cc2O)cc1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Phloretin is a natural dihydrochalcone, originally identified from the apple tree, and is a metabolite of phlorizin. It has numerous pharmacological effects, including anti-inflammatory, antidiabetic, anticancer, antimicrobial, hepatoprotective, neuroprotective and cytoprotective properties. Phloretin is a potent antioxidant, scavenging peroxynitrite (ONOO-) and/or suppressing lipid peroxidation, which contributes to the many biological properties. Phloretin has anti-aging and depigmenting effects, making it also attractive for dermatological research and application. Phloretin attacks a number of molecular targets, modulating different intracellular signaling pathways, including NF-κB, MAPK, Nrf2, and AMPK. It can modulate Ca2+ channels, PKC, GLUTs, SGLTs and other targets. The anticancer potential is based on antiproliferative, proapoptotic, antimetastatic, and antiangiogenic activities.
(1) M.R. de Oliveira; Biofactors 42, 13 (2016) (Review) | (2) B.Y. Choi; Molecules 24, 278 (2019) (Review) | (3) T.P.A. Casarini, et al.; Eur. J. Pharmacol. 889, 173593 (2020) (Review) | (4) K.T. Nakhate, et al.; Nutrients 14, 3638 (2022) (Review) | (5) H.S. Tuli, et al.; Molecules 27, 8819 (2022) (Review) | (6) S. Habtemariam;, Biomedicines 11, 143 (2023) (Review)





