Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
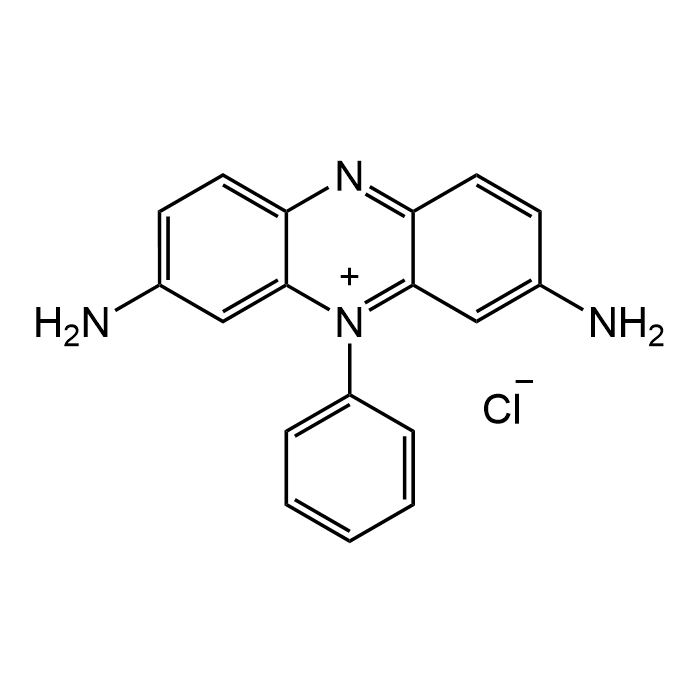
Phenosafranin
| Product Details | |
|---|---|
| Synonyms | Phenosafranine; 3,7-Diamino-5-phenylphenazinium chloride; C.I. 50200; NSC215209; NSC 9855 |
| Product Type | Chemical |
| Properties | |
| Formula | C18H15ClN4 |
| MW | 322.79 |
| CAS | 81-93-6 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥80% (Dye content) |
| Appearance | Green to dark green powder. |
| Solubility | Soluble in DMSO (20mg/ml). |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SOUHUMACVWVDME-UHFFFAOYSA-N |
| Smiles | NC1=CC=C2C([N+](C3=CC=CC=C3)=C(C=C(N)C=C4)C4=N2)=C1.[Cl-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Phenosafranin is a phenazine dye which is an organic chloride salt having 3,7-diamino-5-phenylphenazin-5-ium as the counterion. It has a high binding affinity to triplex RNA compared to the parent duplex form, and binds through intercalation to both forms of RNA. This property makes it useful for staining plant cells and for the determination of hemoglobin, dopamine and serotonin. It binds also to double-stranded DNA. It has a role as a fluorochrome (e.g. to functionalize different reagents), a histological dye and a photosensitizing agent. Phenosafranin has also shown biological properties, such as inhibit nuclear localization of transglutaminase 2 without affecting its transamidase activity. Spectral Data: Absorbance λmax=519nm.
(1) E.J. Moore; Science 77, 23 (1933) | (2) S. Brenner; Biochim. Biophys. Acta 11, 480 (1953) | (3) J.M. Widholm; Stain Technol. 47, 189 (1972) | (4) S.A. Curran, et al.; J. Chem. Phys. 120, 4886 (2004) | (5) I. Saha, et al.; J. Phys. Chem. B 114, 15278 (2010) | (6) I. Saha & G.S. Kumar; Fluoresc. 21, 247 (2011) | (7) W. Liu, et al.; J. Sol. Chem. 40, 231 (2011) | (8) M.F. Broglia, et al.; Photochem. Photobiol. Sci. 14, 407 (2015) | (9) B. Saha & G.S. Kumar; J. Photochem. Photobiol. B 161, 129 (2016) | (10) A.B. Pradhan, et al.; Int. J. Biol. Macromol. 86, 345 (2016) | (11) Y. Furutani, et al.; Amino Acids 49, 483 (2017) | (12) J. Hou, et al.; Sensors 20, 1501 (2020) | (13) K. Rozga-Wijas, et al.; Int. J. Mol. Sci. 22, 13373 (2021)





