Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
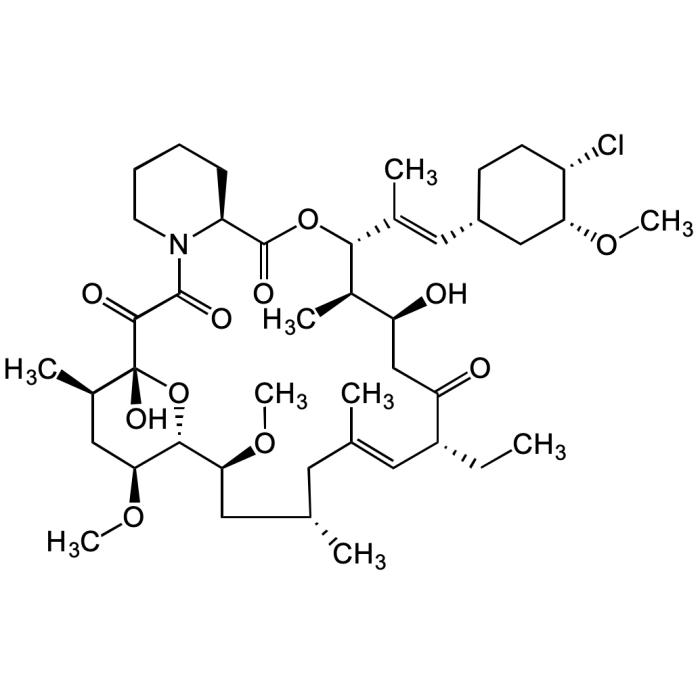
Pimecrolimus
| Product Details | |
|---|---|
| Synonyms | Elidel; SDZ-ASM 981; 33-Epichloro-33-desoxyascomycin |
| Product Type | Chemical |
| Properties | |
| Formula | C43H68ClNO11 |
| MW | 810.45 |
| CAS | 137071-32-0 |
| RTECS | KD4179200 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to beige powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or ethanol (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | KASDHRXLYQOAKZ-XDSKOBMDSA-N |
| Smiles | Cl[C@@H]1[C@@H](C[C@@H](CC1)/C=C(C)/[C@H]([C@H](C)[C@H](C2)O)OC([C@H](CCCC3)N3C(C([C@@](O)([C@H](C)C4)O[C@H]([C@H](C[C@@H](C)C/C(C)=C/[C@@H](CC)C2=O)OC)[C@H]4OC)=O)=O)=O)OC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Pimecrolimus is a immunosuppressive natural ascomycin macrolactam that binds to macrophilin-12 (FKBP-12) and inhibits calcineurin as well as prolyl isomerase (rotamase). Presumably through these actions, it blocks the activation of T cells by allogeneic dendritic cells (IC50=0.55nM) without affecting dendritic cells. Moreover, pimecrolimus suppresses the generation of pro-inflammatory cytokines by T cells, the release of pre-formed inflammatory mediators from mast cells and the activation of eosinophils. These effects support the use of pimecrolimus in countering inflammatory skin diseases, such as atopic dermatitis (eczema) and psoriasis.
(1) D. Bochelen, et al.; J. Pharmacol. Exp. Therap. 288, 653 (1999) | (2) M. Grassberger, et al.; Br. J. Dermatol. 141, 264 (1999) | (3) T. Zuberbier, et al.; J. All. Clin. Immunol. 108, 275 (2001) | (4) F.S. Kalthoff, et al.; Clin. Exp. Immunol. 130, 85 (2002) | (5) K. Rappersberger, et al.; J. Invest. Dematol. 119, 876 (2002) | (6) J.G. Meingassner, et al.; J. Invest. Dermatol. 121, 77 (2003) | (7) F.S. Kalthoff, et al.; Clin. Exp. Immunol. 133, 350 (2003) | (8) J.G. Meingassner, et al.; Br. J. Dermatol. 149, 853 (2003) | (9) D.A. Plager, et al.; Int. Arch. Allergy Immunol. 149, 119 (2009) | (10) Z. Ma & Z. Jiao; Curr. Pharm. Des. 17, 3823 (2011) (Review) | (11) S.G. Danby & M.J. Cork; Br. J. Dermatol. 168, 235 (2013)





