Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
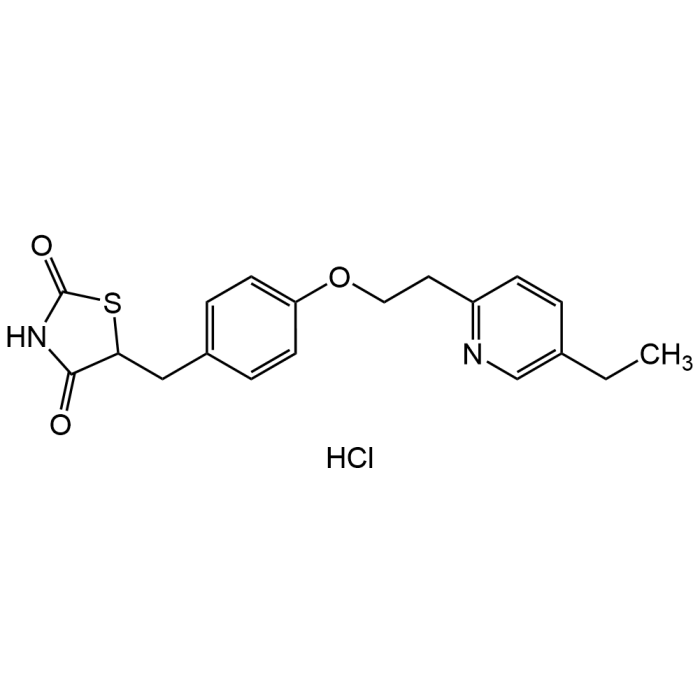
Pioglitazone hydrochloride
As low as
58
CHF
CHF 58.00
In stock
Only %1 left
CDX-P0733-M01010 mgCHF 58.00
CDX-P0733-M05050 mgCHF 232.00
| Product Details | |
|---|---|
| Synonyms | 5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione monohydrochloride; U 72107A |
| Product Type | Chemical |
| Properties | |
| Formula | C19H20N2O3S . HCl |
| MW | 392.9 |
| CAS | 112529-15-4 |
| RTECS | XJ5813440 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO or DMF (both 10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | GHUUBYQTCDQWRA-UHFFFAOYSA-N |
| Smiles | CCC(C=C1)=CN=C1CCOC2=CC=C(CC3SC(NC3=O)=O)C=C2.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Pioglitazone hydrochloride is a proliferator-activated receptor γ (PPARγ) agonist (EC50 = ~500-600nM for both human and murine PPARγ) and thiazolidinedione (TZD) anti-diabetic. Pioglitazone is a selective agonist of the nuclear receptor peroxisome proliferator-activated receptor γ (PPAR-γ) and to a lesser extent PPAR-α. Pioglitazone hydrochloride is usually used to treat type-II diabetes in vivo and has the ability to block hepatic gluconeogenesis. Pioglitazone inhibits pyruvate oxidation and glucose production in hepatocytes when used at a concentration of 10 μM. In vivo, pioglitazone (0.3-3mg/kg per day) reduces hyperglycemia, hyperlipidemia, and hyperinsulinemia in a dose-dependent manner in male Wistar fatty rats. It shos also anti-inflammatory and anti-arteriosclerosis effects.
Product References
(1) Y. Sugiyama, et al.; Arzneimittelforschung 40, 263 (1990) | (2) J.M. Lehmann, et al.; J. Biol. Chem. 270, 12953 (1995) | (3) T.M. Willson, et al.; J. Med. Chem. 39, 665 (1996) | (4) J. Sakamoto, et al.; BBRC 278, 704 (2000) | (5) T.M. Willson, et al.; J. Med. Chem. 43, 527 (2000) | (6) M. Ishibashi, et al.; Hypertension 40, 687 (2002) | (7) P. de Pablos-Velasco; Expert Rev. Cardiovasc. Ther. 8, 1057 (2010) | (8) C. Ao, et al.; Cell Biol. Int. 34, 723 (2010) | (9) H.L. Zhang, et al.; Neuroscience 176, 381 (2011) | (10) Q. Zhao, et al.; J. Neuroinflamm. 13, 259 (2016) | (11) S. Suzuki, et al.; Int. J. Mol. Sci. 17, E2071 (2016) | (12) C.E. Shannon, et al.; FEBS J. 284, 451 (2017)





