Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
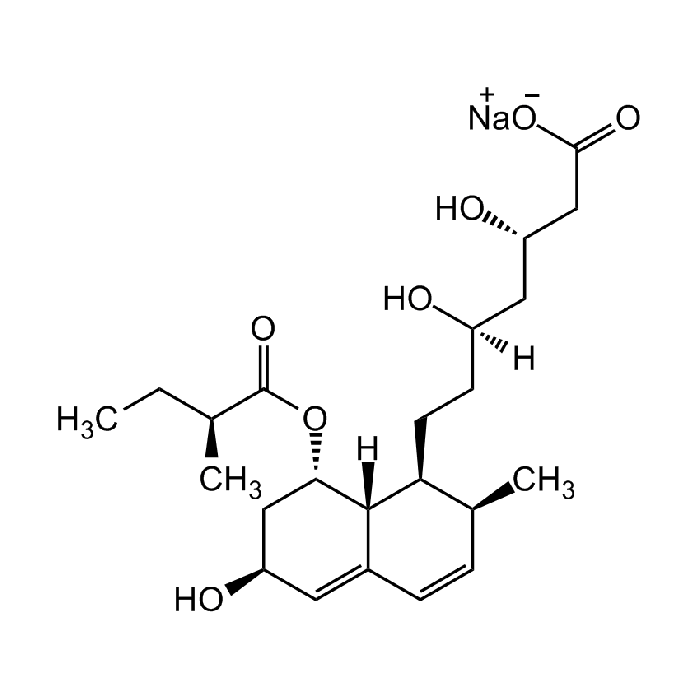
Pravastatin sodium salt hydrate
| Product Details | |
|---|---|
| Synonyms | Eptastatin sodium salt; 3β-Hydroxycompactin; Mevalothin |
| Product Type | Chemical |
| Properties | |
| Formula |
C23H35O7Na |
| MW | 446.51 |
| CAS | 81131-70-6 (sodium salt) | 81093-37-0 (free acid) |
| RTECS | QJ7185000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥ 98% (HPLC) |
| Appearance | White crystalline solid. |
| Solubility | Soluble in water (30mg/ml) or DMSO (30mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VWBQYTRBTXKKOG-BAWPFLGFSA-M |
| Smiles | O[C@H]1C[C@H](OC([C@@H](C)CC)=O)[C@@]2([H])C(C=C[C@H](C)[C@@H]2CC[C@](O)([H])C[C@H](O)CC(O[Na])=O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Pravastatin is a water-soluble, orally effective, competitive inhibitor of Hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase (Ki = 2.3nM), the rate-limiting enzyme in the cholesterol biosynthetic pathway and the target of the statin class of cholesterol-lowering/regulating compounds. Pravastatin potently blocks cholesterol synthesis in vivo (Ki~ 1 nM) and displays cardioprotective properties. Pravastatin reduces LDL cholesterol and triglyceride levels and increase HDL cholesterol in the prevention of cardiovascular disease. Pravastatin also acts as an immunomodulator and as an inhibitor of p21ras isoprenylation.
(1) T. Koga, et al.; Biochim. Biophys. Acta 1045, 115 (1990) | (2) S.M. Singhvi, et al.; Br. J. Clin. Pharmacol. 29, 239 (1990) | (3) A. Corsini, et al.; Pharmacol. Res. 31, 9 (1995) | (4) B.A. Hamelin & J. Turgeon; Trends Pharmacol. Sci. 19, 26 (1998) | (5) M. Tatsuta, et al.; Br. J. Cancer 77, 581 (1998) | (6) W.H. Kaesemeyer, et al.; J. Am. Coll. Cardiol. 33, 234 (1999) | (7) B. Kwak, et al.; Nat Med. 6, 1399 (2000) | (8) J.A. Tobert; Nat. Rev. Drug Discov. 2, 517 (2003) | (9) A.A. Al-Badr, et al.; Prof. Drug Subst. Excip. Rel. Methodol. 39, 433 (2014)





