Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
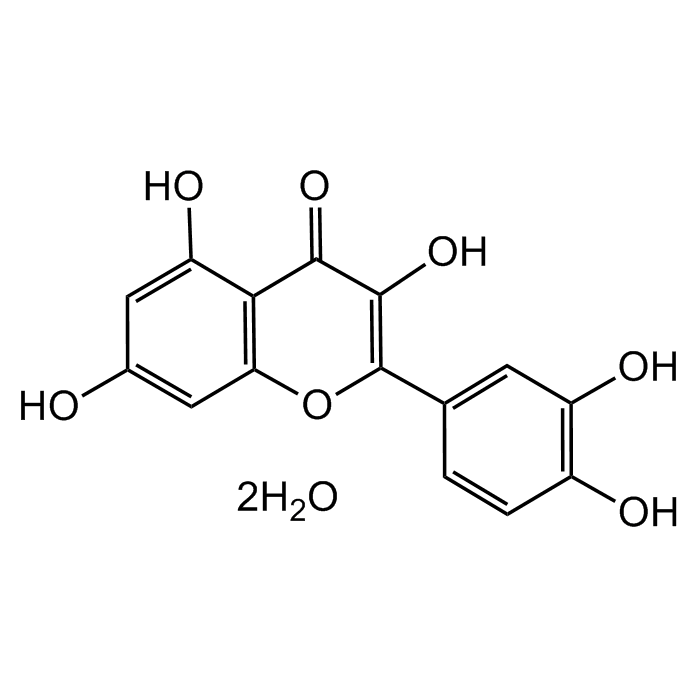
Quercetin dihydrate
| Product Details | |
|---|---|
| Synonyms | 3,3',4',5,7-Pentahydroxyflavone dihydrate; Sophoretin; Meletin; Xanthaurine; Quercetol; Quercitin; Quertine; Flavin meletin; NCI-C60106; NSC9219; NSC9221; CI 75670; CI Natural Yellow 10; BRN0317313 |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H10O7 . 2H2O |
| MW | 338.27 |
| CAS | 6151-25-3 |
| RTECS | LK8950000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Yellow powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or ethanol (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | REFJWTPEDVJJIY-UHFFFAOYSA-N |
| Smiles | OC1=C(C2=CC(O)=C(O)C=C2)OC(C=C(O)C=C3O)=C3C1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Quercetin is a natural multipotent flavonoid with antioxidant, anti-inflammatory, anticancer, neuroprotective, cardioprotective, antidiabetic and antiobesity properties. It has been shown to inhibit NF-κ signaling pathway and the NLRP3 inflammasome activation. It is an apoptosis and autophagy inducer, cell cycle inhibitor, chemosensitizing agent, proteasome inhibitor and activator of SIRT1. It is a pleiotropic kinase inhibitor, including tyrosine protein kinase (Trk), mitochondrial ATPase, cAMP- and cGMP-phosphodiesterases, PI3-kinase activity, phospholipase A2 and protein kinase C (PKC). It is a reversible fatty acid synthase inhibitor.
(1) R. Lupu & J.A. Menendez; Curr. Pharm. Biotechnol. 7, 483 (2006) (Review) | (2) S. Chung, et al.; Arch. Biochem. Biophys. 501, 79 (2010) (Review) | (3) M. Shen, et al.; Anticancer Agents Med. Chem. 12, 891 (2012) (Review) | (4) K. Pallauf & G. Rimbach; Ageing Res. Rev. 12, 237 (2013) (Review) | (5) G.L. Russo; Cancer Treat. Res. 159, 185 (2014) (Review) | (6) S.F. Nabavi, et al.; Food Chem. 179, 305 (2015) (Review) | (7) G. D'Andrea; Fitoterapia 106, 256 (2015) (Review) | (8) Y. Li, et al.; Nutrients 8, 167 (2016) (Review) | (9) D. Kashyap, et al.; Tumour Biol. 37, 12927 (2016) (Review) | (10) D. Barreca, et al.; CNS Neurol. Disord. Drug Targets 15, 964 (2016) (Review) | (11) F. Khan, et al.; Nutrients 8, E529 (2016) (Review) | (12) S. Chen, et al.; Mediators Inflamm. 2016, 9340637 (2016) (Review) | (13) H.M. Eid & P.S. Haddad; Curr. Med. Chem. 24, 355 (2017) (Review) | (14) H. Lim, et al.; Toxicol. Appl. Pharmacol. 355, 93 (2018) | (15) D. Xu, et al.; Molecules 24, E1123 (2019) (Review) | (16) H. Khan, et al.; Biomolecules 10, E59 (2019) (Review)





