Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
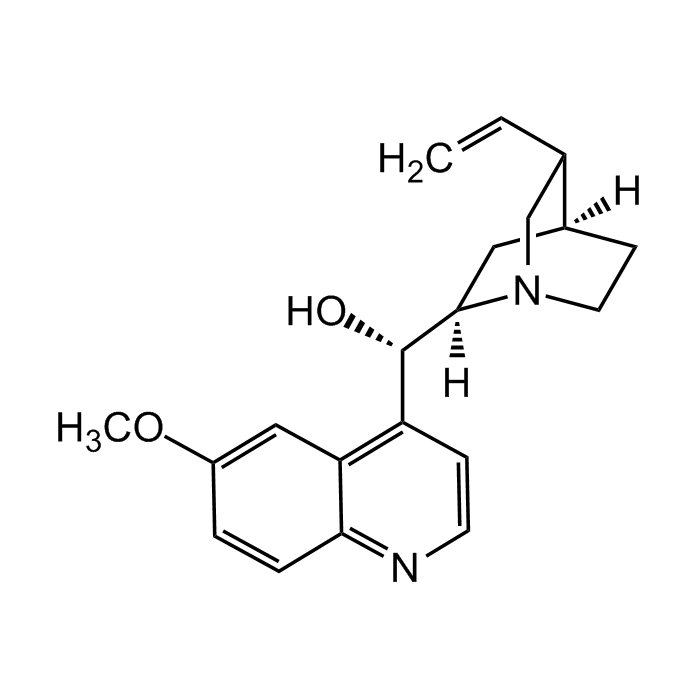
Quinidine (anhydrous)
| Product Details | |
|---|---|
| Synonyms | α-(6-Methoxy-4-quinolyl)-5-vinyl-2-quinuclidinemethanol |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H24N2O2 |
| MW | 324.42 |
| CAS | 56-54-2 |
| RTECS | VA4725000 |
| Source/Host Chemicals | Isolated from plant. |
| Purity Chemicals | ≥99% (Titr.) |
| Appearance | White to faint yellow powder. |
| Solubility | Soluble in DMSO (20mg/ml) or ethanol (5mg/ml). |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LOUPRKONTZGTKE-LHHVKLHASA-N |
| Smiles | O[C@@H](C1=C(C=C(OC)C=C2)C2=NC=C1)[C@]3([H])[N@](C[C@@H]4C=C)CC[C@@]4([H])C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Quinidine is a dextrorotatory stereoisomer of Quinine with anti-malarial, anti-arrhythmic (class 1A), anti-inflammatory, anti-cancer and anti-viral properties. Quinidine acts as a blocker of voltage-gated sodium channels. Inhibition of the Nav1.5 channel is specifically involved in its anti-arrhythmic effects as a class I anti-arrhythmic agent. Quinidine also blocks certain voltage-gated potassium channels (e.g., Kv1.4, Kv4.2, hERG, among others), acts as an anti-muscarinic. Quinidine blocks α1-adrenoceptors (alpha1ARs) and is an inhibitor of cytochrome P450 and acetylcholinesterase.
(1) N.J. White, et al.; Lancet 2, 1069 (1981) | (2) K. Schenck-Gustafsson, et al.; Am. J. Cardiol. 51, 777 (1983) | (3) L. Crevasse; Am. J. Cardiol. 62, 22I (1988) | (4) A Yatani, et al.; Circ. Res. 73, 351 (1993) | (5) J.A. Yao, et al.; J. Pharmacol. Exp. Ther. 279, 856 (1996) | (6) K. Shibata, et al.; Circulation 97, 1227 (1998) | (7) S. Wang, et al.; J. Physiol. 546, 387 (2003) | (8) J.M. Hutzler, et al.; Chem. Res. Toxicol. 16, 450 (2003) | (9) L.A. McLaughlin, et al.; J. Biol. Chem. 280, 38617 (2005) | (10) M.K. Pugsley, et al.; Clin. Exp. Pharmacol. Physiol. 32, 60 (2005) | (11) B. Bozic, et al.; Mini Rev. Med. Chem. 18, 468 (2018) | (12) L. Vitali Serdoz, et al.; Pharmacol. Res. 144, 257 (2019) | (13) Z. Che, et al.; Chem. Biodivers. 17, e1900696 (2020) | (14) Z. Li, et al.; Angew. Chem. Int. Ed. Engl. 60, 11474 (2021)





