Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
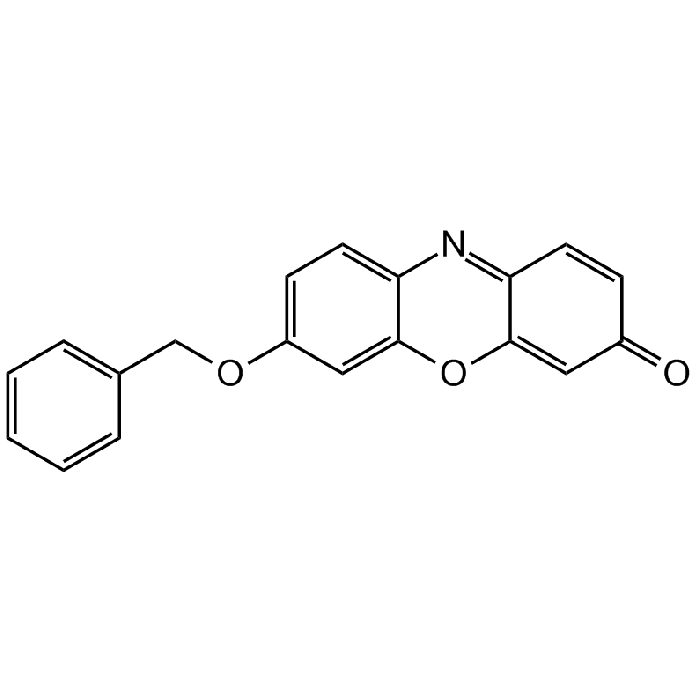
Resorufin benzyl ether
| Product Details | |
|---|---|
| Synonyms | Benzyloxyresorufin; 7-Benzyloxyresorufin; O7-Benzylresorufin; 7-Benzyloxy-3H-phenoxazin-3-one |
| Product Type | Chemical |
| Properties | |
| Formula | C19H13NO3 |
| MW | 303.31 |
| CAS | 87687-02-3 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Orange to red powder. |
| Solubility | Soluble in DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XNZRYTITWLGTJS-UHFFFAOYSA-N |
| Smiles | O=C1C=C2OC3=CC(OCC4=CC=CC=C4)=CC=C3N=C2C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Resorufin benzyl ether (7-Benzyloxyresorufin) is a fluorogenic substrate widely used for monitoring cytochrome P450 (CYP450) activities in cell extracts and solutions. It is the model substrate for 7-Benzoxyresorufin-O-dealkylase (BROD) assays. It is typically used near its apparent Km value of 30 µM to screen the inhibition/activation potential of test compounds, to predict potential drug-drug interactions, or to monitor other CYP450 activities. Cytochrome P450 oxidase is a large number of evolutionary related oxidative enzymes important in animal, plant, and bacterial physiology. Most cytochromes P450 (CYPs) have about 500 amino acids and a heme group at the active site. Upon enzymatic cleavage of 7-Benzyloxyresorufin by CYP450, the fluorescent hydrolysis product resorufin is released and its fluorescence can be used to quantify enzyme activity. Spectral data of Resorufin: λex=571nm, λem=585nm.
(1) R.T Mayer, at al.; Biochem. Pharmacol. 40, 1645 (1990) | (2) D.M. Stresser, et al.; Drug Metab. Dispos. 28, 1440 (2000) | (3) A.B. Renwick, et al.; Xenobiotica 31, 861 (2001) | (4) D.M. Stresser, et al.; Drug Metab. Dispos. 30, 845 (2002) | (5) B.M.A. Lussenburg, et at.; Anal. Biochem. 341, 148 (2005) | (6) J.R. Reed, et al.; Biochem. Pharmacol. 95, 126 (2015) | (7) J. Reinen, et al., J. Biomol. Screen. 20, 1246 (2015) | (8) M.J. Traylor, et al.; Insect Biochem. Mol. Biol. 90, 14 (2017) | (9) L. Rutz, et al.; Ach. Toxicol. 94, 4159 (2020)





